#ScienceSaturday posts share relevant and exciting scientific news with the KAND community. This project is a collaboration between KIF1A.ORG’s Research Engagement Team Leader Alejandro Doval, President Kathryn Atchley and Chief Science Officer Dr. Dominique Lessard. Send news suggestions to our team at impact@kif1a.org.
Recent KIF1A-Related Research
PTP-3 phosphatase promotes intramolecular folding of SYD-2 to inactivate kinesin-3 UNC-104 in neurons
Today we are revisiting the nematode worm model system known as C. elegans! This recent paper is investigating ways in which the transport of synaptic protein vesicles by UNC-104 (C. elegans version of KIF1A) is regulated in nerve cells. In this manuscript, we find three key players: UNC-104, SYD-2, and PTP-3. The interaction between UNC-104 and SYD-2 has been previously established, showing that SYD-2 is a UNC-104 activator that facilitates UNC-104-mediated cargo transport. With this finding in mind, in this study researchers set out to define this mechanism of regulation further by asking the question: if SYD-2 regulates UNC-104, then how is SYD-2 regulated itself? Enter our last player, PTP-3.
PTP-3 is a protein that helps control the addition and removal of post-translational modifications (PTMs). As a reminder, PTMs are specific components that are added to or removed from already existing proteins. This is a lot like adding and removing different “accessories” to a protein structure. This paper revealed that PTP-3 plays an important role in regulating the PTMs on SYD-2, helping to remove a specific “accessory” known as a phosphate. When PTP-3 does so, SYD-2 has a harder time interacting with UNC-104 and thus cannot activate UNC-104. However, when PTP-3 is mutated (rendering it non-functional), SYD-2 is able to increase UNC-104 activation and cargo transport. These types of complex relationships between cellular players are exactly what make the interworking of cells so fascinating! Very rarely do proteins in our cells have one form of an “on/off switch.” Rather, there are multiple ways in which protein function can be modulated at a finely-tuned level. Want to learn more about the nematode worm known as C. elegans? Check out this video below!
Fun Finds!
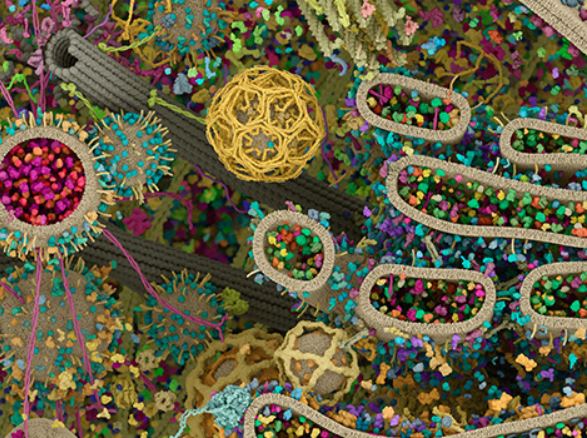
Have you ever wondered what is going on inside of the cellular environment? What kind of cellular setting are kinesin motors like KIF1A navigating through while transporting specific cargo? Enjoy this interactive webpage which highlights how astoundingly crowded the inside of a cell can be! As you navigate through this page here is our challenge to you: can you find a kinesin motor transporting cargo along a microtubule? Have fun!
Rare Disease News
Jim Wilson offers a safer solution to some of the industry’s gene therapy woes
Researchers at the University of Pennsylvania’s Gene Therapy Program released a paper this week on a topic we have been following for months now: the safety of viral vectors for gene therapy. As a reminder, most forms of gene therapy rely on viral vectors to deliver a specific gene of interest to a cell. In other words, if the gene of interest is the contents of a package, the viral vector acts as a delivery box (complete with shipping address!). Like any sort of therapeutic agent, potential side-effects of viral vectors must be identified and diminished to keep patients as safe as possible. This study focused on a special group of neurons called the dorsal route ganglia (DRG). While not entirely clear why, DRG neurons are shown to absorb more viral vector than other cell types. While one might think this is a good thing, researchers have found that this ends up being harmful to DRG neurons and puts them in a state of “cellular stress.” To combat this harmful level of DRG cellular stress, researchers have been able to tactfully modify the amounts of gene expression in DRG cells in primates. To learn more, click the button below! Therapeutic side-effects are sometimes referred to as off-target effects and are an important consideration in therapeutic development. To learn more about off-target effects, check out this video!
Part 3-Gene Therapy: An Industry Perspective
We’re following a four-part series on Global Genes’ RARE Cast podcast about gene therapy. The third episode is a 20-minute conversation with Janet Lambert, CEO of the Alliance for Regenerative Medicine. In this interview Janet discusses the rise of genetic medicine, how gene therapies are changing the landscape for rare diseases, and the challenges developers face in bringing medicines to patients.
“…we’re committed to working with regulators, payers, and legislators to help them understand what the barriers to patient access to these therapies are, and to help work out solutions that work for… the payer community and healthcare systems… as well as benefit the patients that are receiving these therapies.”
Janet Lambert, CEO Alliance for Regenerative Medicine

