#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dylan Verden of KIF1A.ORG summarizes newly published KIF1A-related research and highlights progress in rare disease research and therapeutic development.
KIF1A-Related Research
The Nucleoporin Nup153 Is the Anchor for Kif1a during Basal Nuclear migration in Brain Progenitor Cells
One of the reasons our animal models are so crucial for the treatment of KAND, even as we investigate potential therapeutics, is that we still don’t know all the roles KIF1A may play in the body. Uncovering these roles informs our knowledge of KAND symptoms, progression, and treatment mechanisms. In this week’s pre-print*, researchers including Research Network members Dr. Aditi Falnikar and Dr. Richard Vallee, investigated how a protein called Nup153 anchors KIF1A
Cell Division and Differentiation
As a vertebrate grows from a fertilized egg to a complete body, specialized cell types must develop to carry out different functions. We didn’t start out with so many different types of cells in our brain – many types of neurons, stem cells, and glia in the brain’s cortex developed from common cell type called radial glial progenitors.
These cells are present in embryos, where they populate the brain by dividing: When a radial glial progenitor (RGP) divides it can make two radial glial progenitor copies to increase cell number, or it can make a single copy as well as a mature neuron. This process can have drastic effects on the number of cells in the brain, as well as their type and organization.
As the RGP divides, the nucleus that contains all of the cell’s genetic information moves; the nucleus begins toward the top of the cell as it begins dividing, and moves downward during the process. Previous work from this group found that KIF1A plays a role in the downward migration of the nucleus during RGP division.
The Study
The authors investigated Nup153, a protein that forms binding complexes on the surface of the nucleus. Nup153 was chosen from several similar proteins in its family based on the likelihood of KIF1A interactions. To investigate these proteins, the authors used RNA interference to silence Nup153 and KIF1A. This technique only knocks out the protein in some cells, so researchers can also investigate interactions between healthy and knockdown cells.
Similar to KIF1A knockdown experiments, knocking down Nup153 prevented the cells’ nuclei from moving downward. Maturation of neurons also stalled in cells lacking either Nup153 or KIF1A.
By using snips of the KIF1A and Nup153 proteins, the authors were able to identify how Nup153 binds to KIF1A’s stalk region to anchor it to the nucleus, supporting proper cell movement and division.
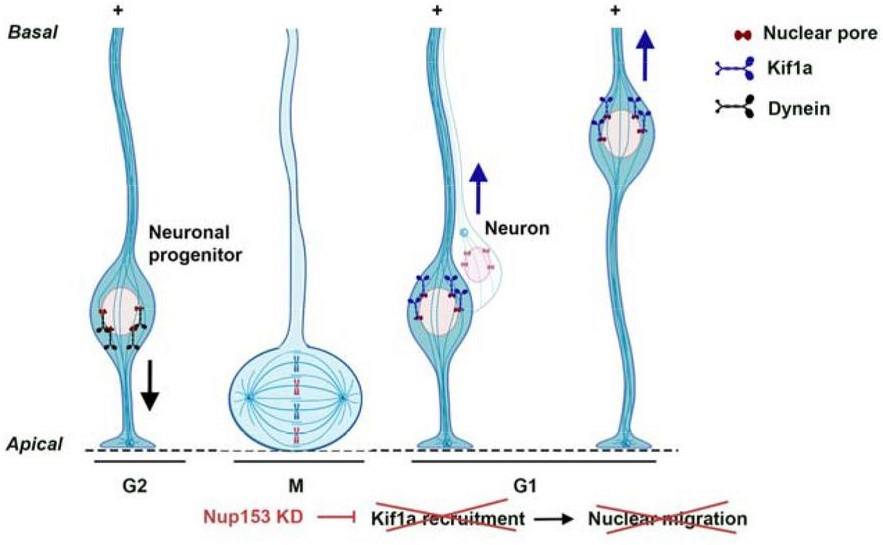
There were also discrepancies between Nup153 and KIF1A knockdowns that are worth further investigation: For example, Nup153 knockdown, but not KIF1A knockdown, caused RGPs to stall during cell division, indicating that Nup153 could be regulating other proteins at the nucleus.
On the other hand, cells without KIF1A disrupted migration in neighboring healthy cells, which was not observed after Nup153 knockdown, raising the question of how KIF1A impacts communication between cells.
As we learn more about KIF1A’s role during embryonic brain development, we can take that knowledge back to our other models, and account for this complexity as we try to find the right therapeutics for KAND.
If you want to hear more about this work, check out Dr. Falnikar’s recent talk at our 2023 conference!
*What’s a pre-print? Check out this #ScienceSaturday post to learn more.
Rare Roundup
A broad genetic test saved one newborn’s life. Research suggests it could help millions of others
We’ve spoken often about the massive opportunities to treat disorders with increased access to newborn genetic screening, including massive screening projects in the United States and United Kingdoms. With our increased understanding of genetic disorders, widening the screening bottleneck is an investment that have huge implications for disease treatment, providing newborns with a jumpstart to better health. This week we want to share a patient’s perspective on newborn screening with an article covering Brynn Schulte, who was able to get a diagnosis thanks to a clinical gene screening.