#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dylan Verden of KIF1A.ORG summarizes newly published KIF1A-related research and highlights progress in rare disease research and therapeutic development.
KIF1A-Related Research
Phosphatidylinositol 3,5-bisphosphate facilitates axonal vesicle transport and presynapse assembly
Imagine a transport company that sends truck shipments of bricks to a construction company; the bricks will be used later, but for now they’ll be stored in a warehouse that serves as the hub for operations. This warehouse also happens to be made of bricks, leading you to wonder if these trucks also played a critical role in delivering the materials for the initial warehouse.
Similarly, KIF1A is associated with transporting cargo to neuronal synapses, the structures through which neurons communicate with one another. Like the warehouse, synapses also have to be constructed before they can act as a viable delivery site. This construction is a crucial part of neurodevelopment, and a lack of material can prevent the growth of new synapses. But what regulates the delivery of materials at these early stages?
In this week’s article, researchers investigated KIF1A’s role in the delivery of synaptic building blocks, and its regulation by the lipid (fat molecule) PI(3,5)P2.
Precursor Vesicle Cargo
Synapses are complex structures whose assembly requires many different pieces. To transport each piece individually would be very inefficient, so molecules going to the same destination are often packaged into containers we call vesicles. Vesicles undergo complex regulation to determine their ultimate destination, and scientists categorize them according to factors like size and the presence of specific molecules that act like shipping labels. But because many types of cargo can coexist in the same vesicle, there is a lot of complexity to vesicle biology that is still being uncovered.
Why is it important to think about specific cargo? Of the many types of KAND-related KIF1A mutations, several are located in regions that control KIF1A’s recruitment and cargo-binding. Because these precursor vesicles are responsible for building synapses during development, understanding these mechanisms can give insight into the neurodevelopmental aspects of KAND.
The Study
To investigate precursor vesicle trafficking, the researchers used neurons derived from human induced pluripotent stem cells (iPSCs). The authors were able to track the movement of vesicles, determine their size, and identify some of their proteins, including Active Zone Proteins and Neurexin1b, which are crucial for synaptic function.
A protein associated with precursor vesicle trafficking is ARL8, which can exist in two forms, ARL8A and ARL8B. Knocking down these proteins prevents the transport of the vesicles, and as a result synapses don’t form properly. In a screen for proteins that likely associate with ARL8, the researchers identified KIF1A, and investigated its role in precursor vesicle transport.
Knocking out the KIF1A gene, knocking down KIF1A production, or replacing KIF1A with a mutant version (G251A) that couldn’t walk all copied the effects of the ARL8 knockdown, pointing to a shared mechanism.
However, the authors also noted that certain pathways could increase precursor vesicle transport even when ARL8 was absent; these pathways seemed to rely on the production of lipids (fat molecules) called PI(3)P2 and PI(3,5)P2. These lipids can be found on the surface of precursor vesicles, and were found to act like a tether between precursor vesicles and a specific section of KIF1A called the pleckstrin homology (PH) domain; removing the PH domain, or preventing the production of PI(3)P2/PI(3,5)P2 lipids, prevented transport of cargo.
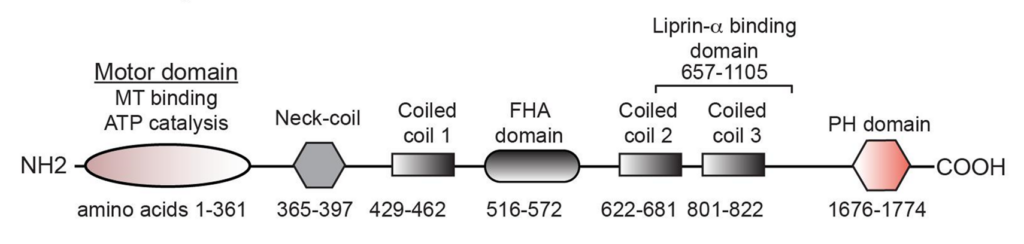
Together, these findings indicate that ARL8 and PI(3)P2/PI(3,5)P2 lipids are both regulators of KIF1A that impact its ability to build synapses during neurodevelopment. Several KAND-related mutations have been identified in the PH domain, presenting as hereditary and sensory neuropathy (HSAN KAND) or spastic paraplegia phenotypes; investigating its role in KIF1A biology can help us better understand and address KAND progression and subtypes.
Rare Roundup
Rare diseases and the role of primary care
Just like there is not likely to be one single silver bullet for KAND, there is no one-stop-shop for healthcare for our community – KAND is a complex neurological disorder, and its management requires the coordination of several specialties and levels of expertise. But to identify a disorder, and line up those resources, requires that a medical professional recognizes the need. For many patients, that professional is their primary physician.
With 7,000-10,000 known rare diseases, it’s not possible to be an expert in all, but being on the lookout for potential signs, and knowing which steps to take next, can significantly shorten a patient’s diagnostic journey. This resource describes the importance of primary care in managing rare diseases, and provides some guidance on navigating potential rare disease diagnosis.
We know that our community benefits from a slew of doctors who have gone above and beyond to understand and help manage KAND cases; we also know that many times KAND families come to doctors as KAND experts. We’re grateful for guides like this that bridge the gap between symptoms and diagnosis.

