#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dylan Verden of KIF1A.ORG summarizes newly published KIF1A-related research and highlights progress in rare disease research and therapeutic development.
KIF1A-Related Research
A Sideroflexin/Connexin adaptor ties kinesin-3 to mitochondria in neurons
If your grocery store was out of both apples and oranges, you might think there are two shortages: apples and oranges. But another possibility is that something happened to the truck that delivers fruit to the grocery store; the fruit is ready, it just hasn’t gotten to the right place.
Similarly, KIF1A is responsible for carrying many types of cellular cargo, which is why KIF1A mutations can cause such a wide variety of symptoms. In this week’s pre-print, researchers found evidence for KIF1A’s role in transporting a very important type of cargo: Mitochondria.
You may have heard the phrase that mitochondria are the “powerhouse of the cell”: This describes their ability to produce ATP, the molecule that provides energy for the metabolism of all known living cells.
Because of their ATP production mitochondria can support areas with high metabolic demand, like the axons and dendrites of neurons. When mitochondria can’t reach these destinations it can disrupt cellular metabolism and cause neurodegeneration.
Part of how KIF1A picks its cargo is by interactions with adaptor proteins: Like hitch adaptors on a semi truck, they help attach to specialized cargo containers.
The authors investigated two adaptors: SFXN-1.2, which was found to impact mitochondria movement, and CX32, a known binder of SFXN-1.2 and KIF1A. In their C. elegans worm model, SFXN-1.2, CX32, and UNC-104 (the worm’s version of KIF1A) were found to interact at mitochondria. Knocking down either SFXN-1.2 or KIF1A decreased the movement of mitochondria, and knocking down both had an even greater impact, indicating that they cooperate to transport mitochondria. CX32 likely regulates this cooperation, as knocking down CX32 weakened the connection between SFXN-1.2 and KIF1A.
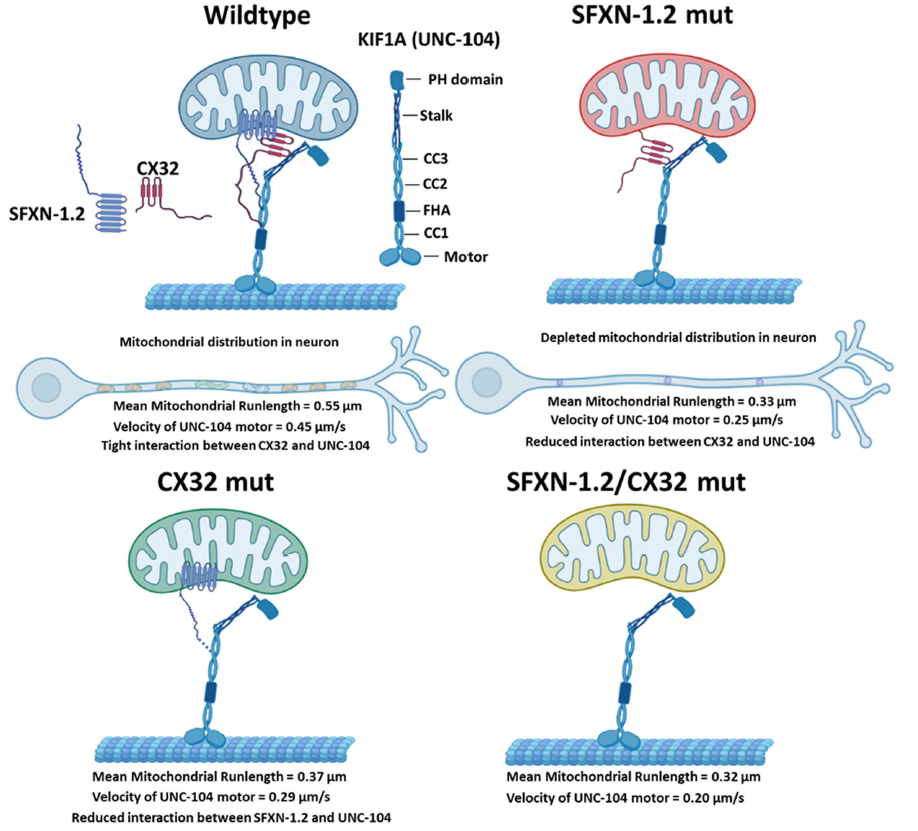
In the figure above we see KIF1A, SFXN-1.2, and CX32 teaming up to attach to a mitochondrion, as well as the consequences of knocking down the adaptor proteins. Note that the mitochondrial runlength and KIF1A velocity both decrease without adaptor proteins.
The attachment points in the figure are no accident either; the study also found that SFXN-1.2 interacts with three regions of KIF1A: the Coiled Coiled Domains 2 and 3 (CC2 and CC3), and the Stalk. These regions aren’t characterized as well as KIF1A’s motor domain, but play roles in forming KIF1A dimers and binding to cargo. Investigating mitochondrial dynamics in KAND-related mutations in the CC2, CC3 and the Stalk domains may help us learn more about causes of, and therapeutic targets for, KAND symptoms.
*What’s a pre-print? Check out this #ScienceSaturday post to learn more
Rare Roundup
FDA sets decision dates for Vertex, CRISPR gene editing drug
It feels like CRISPR and gene editing conversations have been going on for a long time, but using them as treatments for human diseases is still in its early stages; this is why the FDA’s decision date for exa-cel, a CRISPR-based gene therapy for sickle cell disease and beta thalassemia, has garnered so much attention. The FDA will issue two separate decisions, one for each disease application: Sickle cell disease in December of 2023, and beta thalassemia in March of 2024.
Both sickle cell disease and beta thalassemia are genetic disorders that compromise hemoglobin, the oxygen-binding protein found in our red blood cells. In clinical trials, stem cells derived from patient blood samples were collected, and treated with exa-cel to increase production of fetal hemoglobin. These treated cells were then infused back into the patients, with the goal of them maturing into a population of healthy blood cells. Exa-cel met its endpoints in both clinical trials: treated patients needed less blood transfusions, and patients with sickle cell disease had less bouts of extreme pain.
Because we can’t take neurons out, edit them, and put them back in, exa-cel’s administration method may not be as useful for neurological disorders like KAND. But this landmark in gene editing regulation will tell us more about the FDA’s approach to gene therapies.

