#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dominique Lessard and Dr. Dylan Verden of KIF1A.ORG summarize newly published KIF1A-related research and highlight progress in rare disease research and therapeutic development.
What Is KIF1A?
As we are one month out from our community conference on August 13th, we want to acknowledge how much that community has grown in the last year. As new families join us in our relentless search for treatments and cures for KIF1A Associated Neurological Disorder, we invite you to learn more about KIF1A and how it functions in our bodies from our Chief Science Officer Dr. Dominique Lessard.
Rare Roundup
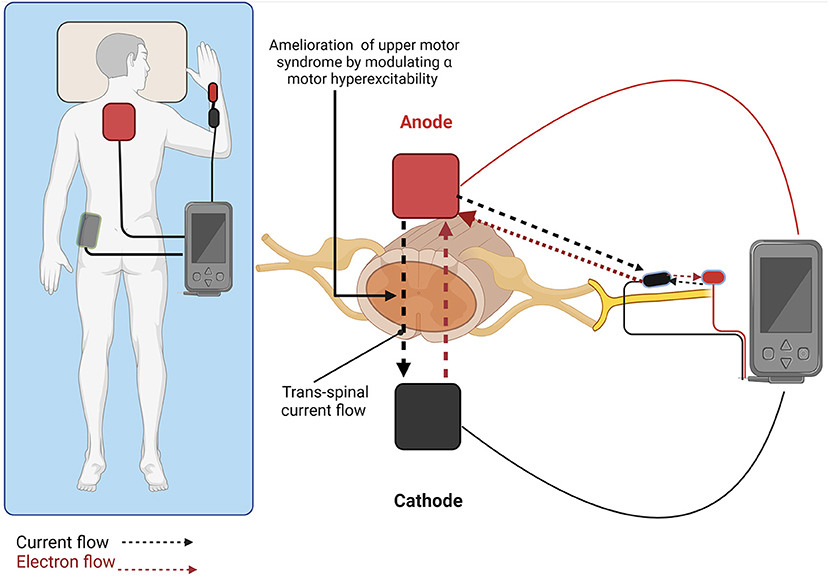
Trans-Spinal Direct Current Stimulation in Spasticity: A Literature Mini-Review
There are many ways to treat neurological disorders. Most of us are familiar with pharmacological or chemical interventions: drugs are taken to change the way our neurons operate. We can also intervene physically: surgical interventions remove or damage dysfunctional cells to improve symptoms. Our nervous system operates through electrical currents, and manipulating those currents can encourage and train proper circuit function. Cardiac pacemakers and deep brain stimulation are examples of life-changing electrical therapies, but their surgical implantation can be quite invasive. This week’s article reviews a newer electrical therapy for spasticity that can be worn on the skin: Trans-Spinal Direct Current Stimulation (tsDCS).
How does it work? As its name implies, tsDCS operates by sending stimulating currents across the spinal cord. The spinal cord carries sensory, motor, and pain signals, each of which can be modulated by electrical activity. Electrodes are placed on the skin, and emit electrical currents that change the activity of spinal cord neurons–the effects depend on the placement of the electrodes, and stimulation features like intensity and frequency.
Because the nervous system is always adapting, the benefits of tsDCS can continue after the stimulation is turned off. Furthermore, stimulation parameters can be changed flexibly without invasive procedures.
Morales-Quezada Leon et al. Trans-spinal Direct Current Stimulation (tsDCS) in Spasticity: a literature mini-review. Frontiers in Stroke.
DOI: 10.3389/fstro.2022.921450
What conditions are being studied? It’s important to note that tsDCS is an emerging therapy that is still being studied. As the authors note, it has been found to impact multiple symptoms in clinical trials:
- Spasticity in hereditary spastic paraplegia and stroke patients
- Neuropathic pain in multiple sclerosis
- Muscle strength in spinal cord injury
Could this help with KAND? These studies open the possibility of creating customized electrical interventions for treating motor dysfunction. However, there are some considerations that are important for safety:
- The studies described here were all performed in adults, and further work needs to be done to understand pediatric use.
- While tsDCS has been tested for hereditary spastic paraplegia, KAND can also cause epilepsy, which is ultimately an electrical disorder. Any interactions between current stimulation and seizure-like activity are crucial to assess.
Researchers identify substantial lack of racial and ethnic data in studies of rare genetic disorder
In diagnosing diseases and developing therapeutics, comprehensive data is crucial. This is especially true for rare diseases, where every data point counts. Genetic disorders occur in all populations, but this isn’t necessarily represented in medical literature. By ignoring demographic data, medical research can underserve vulnerable populations. Researchers of Hereditary Hemorrhagic Telangiectasia (HHT) at Johns Hopkins Medical School reviewed clinical studies from recent years and found that racial and ethnic data was not reported, which prevents analysis of efficacy and safety of therapeutics for non-Caucasian patients. Holistic representation is a crucial step toward countering bias to develop inclusive diagnostics and effective treatments for all rare disease patients.

