#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dylan Verden of KIF1A.ORG summarizes newly published KIF1A-related research and highlights progress in rare disease research and therapeutic development.
KIF1A-Related Research
A two-kinesin mechanism controls neurogenesis in the developing brain
As we bring in the New Year with this week’s #ScienceSaturday we’re excited to share research from long-time Research Network members in the Vallee Lab, who have investigated how KIF1A and another kinesin called KIF13B regulate the generation of neurons in the developing brains of rodent embryos.
We all start as clumps of similar cells: As these cells reproduce, they take on different features according to biological cues; some become skin, some become bone, and so on. In the brain, cells called Radial Glial Progenitors (RGPs) give rise to neurons and other types of cells.
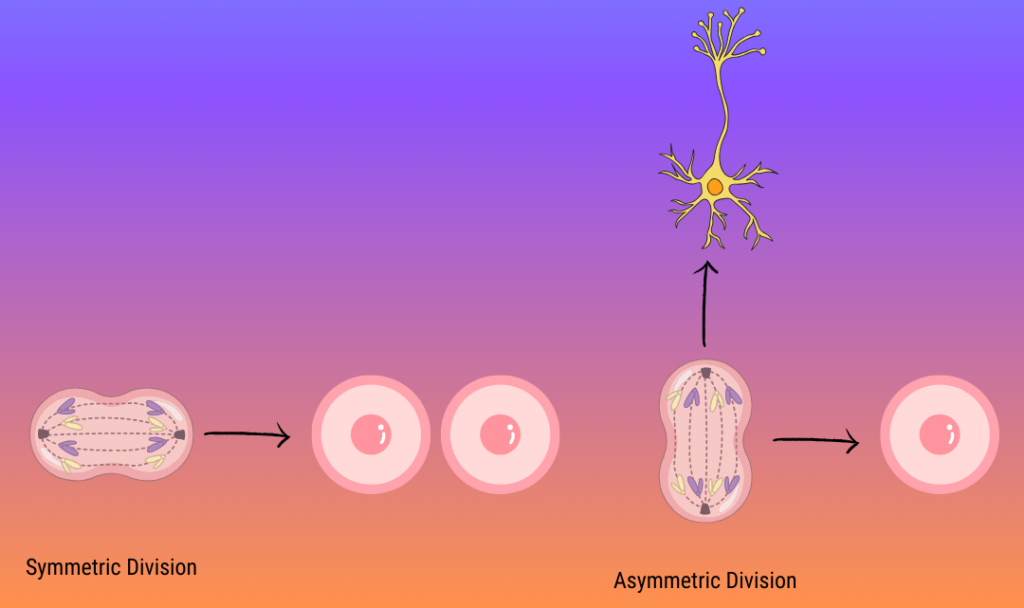
This process is regulated so we end up with the right number of neurons, which we can imagine with some simplified math:
If you start with one progenitor and need eight neurons, it isn’t efficient for that progenitor to make a single neuron eight times. Instead, the first RGP can divide into two RGPs, which can divide into 4, then 8: this is called symmetric division, and it makes sure that there are enough RGPS to create other brain cells. RGPs can also exit this cycle by dividing into an RGP and a neuron, a process called neurogenesis. Neurogenesis can be a direct process, but RGPs can also create intermediate cells that make neurons later on.
Controlling symmetric and asymmetric division is crucial for populating the brain with the right cells in the right numbers. Previous studies have found that when KIF1A is knocked down, RGPs favor symmetric divisions, preventing the timely creation of neurons in the developing brain. But the role of KIF13B in brain development was unknown.
The Study
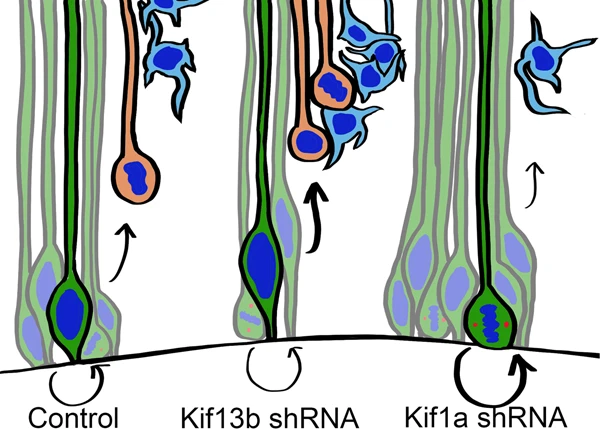
KIF1A and KIF13B have similar, but not identical, structure, leading to the question of how they might impact cell division. To test this, the investigators knocked down KIF1A or KIF13B and tracked brain development in rats and mice.
They found that knocking down KIF13B caused a reduction of RGPs and an increase in neurons: More intermediate cells were born earlier to create neurons, but less migrated to their proper positions. This could indicate more asymmetric divisions, causing RGPs to create neurons early without boosting their own numbers first.
In contrast, knocking down KIF1A created more progenitors, and less neurons, indicating that KIF1A is crucial for generating neurons during brain development.
So why would two motor proteins that walk along microtubules impact the types of cells that are made? As one cell turns into two, its DNA must be replicated and then pulled apart, so that when the cell divides each has all the DNA it needs to function. The strands that pull the DNA apart are composed of microtubules, and kinesins like KIF1A and KIF13B can impact the direction the strands pull in. Depending on that direction, the new cells may be exposed to the same cues (symmetric division) or different cues (asymmetric division).
We often receive questions from our community about symptom progression, which symptoms treatments are likely to help, and whether treatments will work after a certain age. There’s no simple answer; the disease can vary depending on mutation or even individual. But studies like these that uncover roles for KIF1A at different times in development can help us get closer to better answers.
Want to see more research from the Vallee Lab? You can watch our 2022 interview with Dr. Richard Vallee, and Dr. Aditi Falnikar’s presentation from our 2023 KAND Conference.
Rare Roundup
FDA issues final guidance on rare disease drug development
As we continue to explore new therapeutic avenues for treating KAND, it is crucial to think about the regulatory steps those therapies will face. With feedback from rare disease patients, advocates, and drug developers, the FDA has release a finalized guidance document on rare disease drug development. The development of this guidance has taken several years, and the final version incorporates many aspects that are important for our community, including information of safety and manufacturing, and considerations for pediatric study participants. We look forward to reviewing this new guidance and incorporating it into our approach to find better treatments for KAND.

