#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dylan Verden of KIF1A.ORG summarizes newly published KIF1A-related research and highlights progress in rare disease research and therapeutic development.
KIF1A-Related Research
Presynaptic perspective: Axonal transport defects in neurodevelopmental disorders
When you get caught in a traffic jam, there can be any number of causes: there could be a car accident, an obstacle in the road, or unsafe weather conditions.
But the signs are familiar: Sudden braking, hazard lights, vehicles crowding together and trying to squeeze past one another.
The consequences are similar too: people don’t get where they need to go, deliveries are late, and there’s a potential for additional accidents.
Similarly, disorders that disrupt cargo transport in neurons can have significant overlap; the genes involved may directly interact, or they may have similar effects on long-term neuronal health.
By looking at the players involved in cargo trafficking and the diseases they cause, we may be able to identify new therapeutic opportunities. In this week’s Review Article, researchers describe genetic neurodevelopmental disorders caused by axonal transport defects, including KAND.
Notably, the paper describes four different KAND phenotypes, reflecting disease diagnostic variability:
- Autosomal Dominant NESCAV with seizures and intellectual disabilities
- Autosomal Dominant/Recessive SPG30 with spastic gait
- Autosomal Recessive HSN2C Sensory Neuropathy with muscle weakness
- Hemorrhagic Shock and Encephalopathy Syndrome with severe brain atrophy
The review also describes neurodevelopmental disorders caused by other transport-associated proteins, highlighting that KIF1A is just one of several kinesins whose mutations cause familiar neurodevelopmental symptoms like epilepsy, developmental delay, and sensory neuropathy.
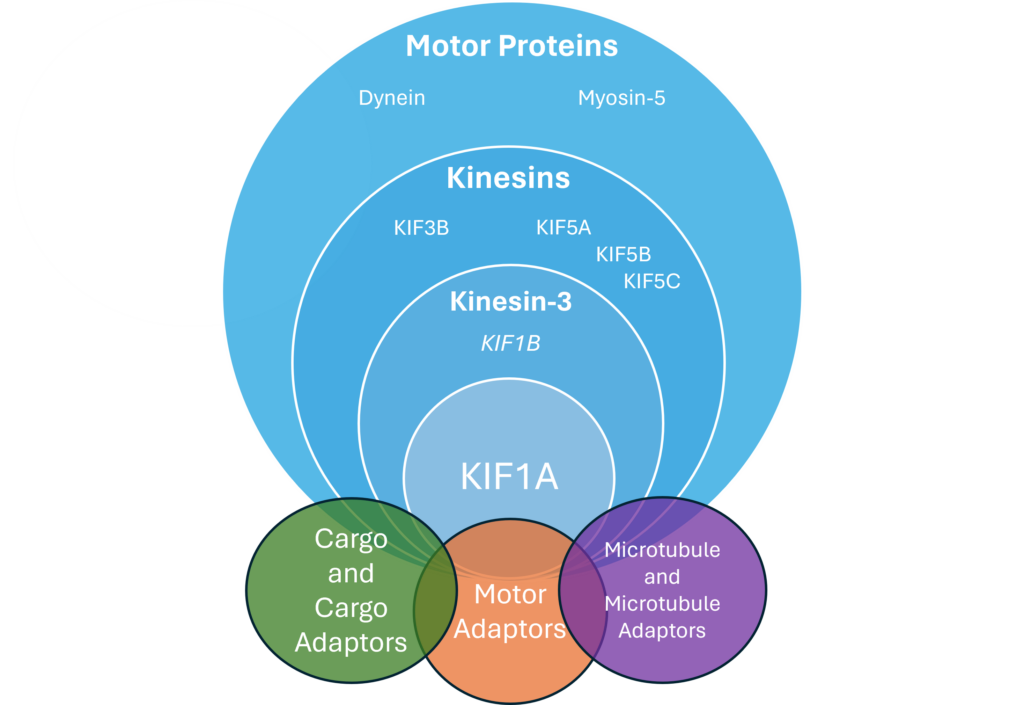
Mutations in interacting systems like KIF1A cargo or microtubule proteins can cause many similar symptoms. In fact, the authors mention several interacting pathways:
- DENN/MAD proteins that help KIF1A bind RAB3 cargo
- Arl8 relieves autoinhibition so KIF1A can carry cargo
- GTP-tubulin only weakly binds to KIF1A, allowing it to release cargo at synapses.
As we continue to define refine our knowledge about KIF1A, it is also helpful to zoom out and see similarities and differences between KAND and other neurodevelopmental disorders so we can find common solutions and improve axonal road conditions.
Rare Roundup
KAND parents present at Dr. Shi’s Biology Course at Stanford
To create research and clinical environments that serve the needs of rare disease patients, it’s important that researchers and clinicians hear patient perspectives, especially during their training; early career scientists play a major role in creating new projects, and early interactions with patients can inform their work for decades to come.
Last year Dr. Rebecca Shi, a professor at Stanford University, designed a class with this in mind: Dr. Shi’s students have been studying KIF1A mutations using the C. elegans worm model, which is transparent and can be genetically modified with relative ease. This allows them to learn more about how specific mutations change KIF1A function, but Dr. Shi wanted to connect these simplified systems to human patients.
This week Katie Bewarder and Lisa Schueneman, parents of Beau and Colbie, presented on their experiences to Dr. Shi’s class for the second year in a row. They highlighted the complexity of KAND, as well as moments of brightness and challenges that Beau and Colbie face. The students had fantastic questions about families’ interactions with the medical community, treatment goals, and patient-centered research.
These experiences can be invaluable in shaping the outlook of early career researchers, and normalize patient families as experts and educators in their own right. A huge thanks Dr. Shi for designing this course, and to Katie and Lisa for taking the time to teach us all.

