#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dominique Lessard and Dr. Dylan Verden of KIF1A.ORG summarize newly published KIF1A-related research and highlight progress in rare disease research and therapeutic development.
KIF1A Research: From the Archives
Polarity of Neuronal Membrane Traffic Requires Sorting of Kinesin Motor Cargo during Entry into Dendrites by a Microtubule-Associated Septin
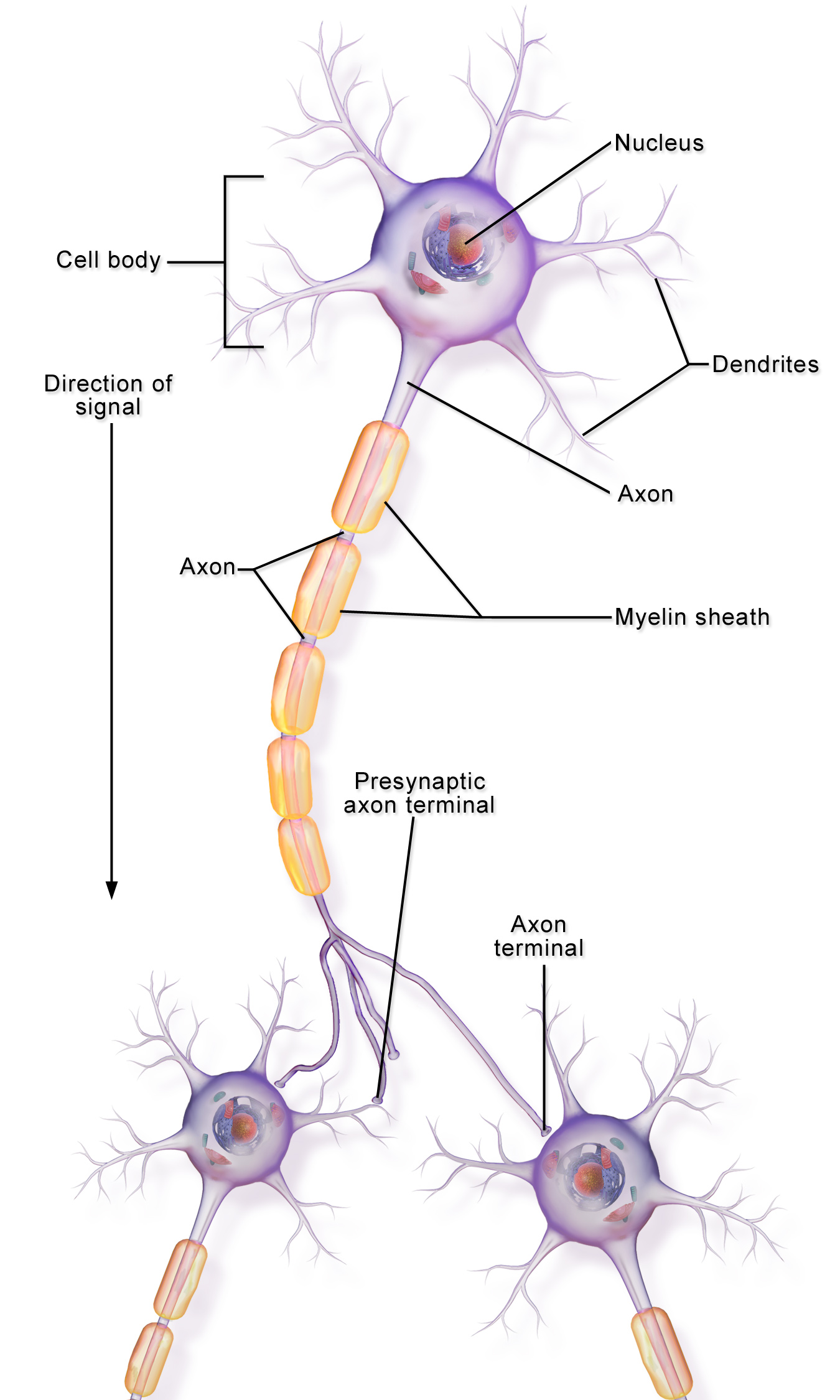
Neurons have complex morphology, and the microtubule roads along which KIF1A proteins travel exist across neuronal compartments. Different cargo needs to be delivered to the neuron’s dendrites and axons, for example, and when cargo enters the wrong compartment it turns around. But what systems exist to make sure the right kinesins show up in the right parts of the neuron? This week’s paper from 2018 explores a checkpoint that exists in neuronal dendrites, allowing KIF1A through while turning another kinesin, KIF5A, back toward the cell body.
Septins are proteins that lie along microtubules, modifying the way other proteins travel along them. SEPT9 is a septin enriched at the boundary of the cell body and the dendrites. SEPT9-coated microtubules caused KIF5A to pause and let go more often, preventing entry into the dendrites. Knocking down SEPT9 expression caused KIF5A-associated cargo, which should go to the axon, to end up in the dendrites instead!
On the other hand, SEPT9-coated microtubules increased KIF1A’s velocity and landing rate, and knocking down SEPT9 caused KIF1A to cluster in the cell body, unable to enter the dendrites. This shows that SEPT9 gives KIF1A a “go” signal to enter dendrites!
How does SEPT9 tell KIF5A to stop and KIF1A to go? It recognizes subtle differences in part of their motor domains called the L12 loop. Swapping the L12 loop between KIF1A and KIF5A changed the way both kinesins localized in the cells. Understanding how to stop or ease KIF1A transport in the cell may help find strategies to counteract KIF1A dysfunction.
Rare Roundup
A Guide To Next Steps After You Receive A Diagnosis
If your family has recently received a genetic diagnosis, Global Genes has a helpful article that explains the basics of genetic test results and questions to ask your healthcare providers to determine your next steps. If your genetic test results includes the KIF1A gene, check out our Newly Diagnosed page for information on KIF1A and KIF1A Associated Neurological Disorder.
“Receiving a rare disease diagnosis after genetic testing can be overwhelming. You may feel relief that you finally have a name for the disease. At the same time, you could be fearful, grieving, angry, or shut down. You may be referred to a specialist who has in-depth knowledge of your condition, or need to be seen at a major medical center that offers specialized care. It’s important to come to conversations about your care prepared.”
Global Genes
Francis Collins Urges Gene Therapy Community to Scale Efforts to Tackle Rare Diseases
In the United States, President Biden’s chief scientific advisor recently discussed progress and hurdles in gene editing therapies at the American Society of Gene and Cell Therapy (ASGCT) Conference. Francis Collins, MD, PhD, has a long history of studying diseases and developing animal models, and has encouraged public-private partnerships to make gene therapies more widely viable. The end of last March saw the first gapless sequence of the human genome, providing a massive boon to rare disease diagnostics. But Collins spoke to the need for systematizing gene editing solutions so we don’t need to go back to the drawing board for each rare disease: “Of nearly 7,000 genetic disorders, only 500–600 have an FDA-approved therapy.” To improve these numbers, we need to improve delivery of gene editing tools so they effectively provide tissue-specific cures while minimizing the number of treatments needed.
Connecticut Establishes a Permanent Rare Disease Advisory Council
Connecticut recently passed House Bill 5500, making it the 23rd state to adopt a permanent Rare Disease Advisory Council (RDAC) thanks to sustained efforts by the National Organization of Rare Disorders (NORD). The RDAC will serve as a liaison through which rare disease stakeholders can make recommendations to policymakers. Empowering the collective voice of rare disease patients, clinicians, and researchers is a crucial step toward systemic changes in therapeutic development and accessibility.

