KIF1A.ORG’s 18th Research Roundtable meeting, “The mechanochemistry of bidirectional axonal transport,” was presented by Dr. William Hancock from Pennsylvania State University. Read on to learn more about how the Hancock Lab is advancing our understanding of KIF1A function.
Attendance
21 RESEARCHERS, CLINICIANS, & BIOTECH REPS

15 RESEARCH INSTITUTIONS & INDUSTRY PARTNERS/ORGS
4 KIF1A.ORG REPS
Who Is Dr. Hancock?
Dr. William Hancock is a Professor of Biomedical engineering at Penn State University who has been studying kinesins for almost 30 years, with an emphasis on KIF1A research for 12 years. His research focuses on how kinesins coordinate their steps and how they compete with other motors proteins. Dr. Hancock has had a very productive year, with multiple studies investigating KIF1A either published or in review. You can learn more about Dr. Hancock and his work in this interview.
Summary
“The mechanochemistry of bidirectional axonal transport”
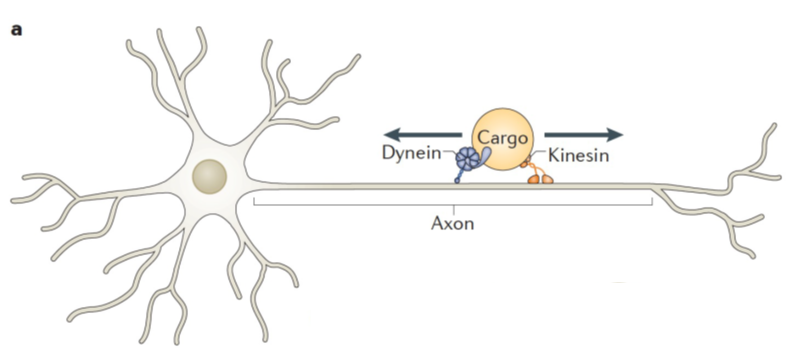
- Cellular cargo is often bound by multiple motor proteins that move in opposite directions on axonal microtubules, which we call bidirectional transport. These motor proteins are responsible for delivering cargo to the right place at the right time within the cell. One standing question in the field of kinesin research is: how do teams of competing motors determine the cargo’s final destination?
- Rather than constantly fighting to move cargo in a “tug-of-war”, competing motors bound to the same cargo may be regulated by other proteins that impact which motor’s turn it is to actively transport the cargo.
- KIF1A is superprocessive, meaning it moves quickly and carries cargo for long distances. Interestingly, while moving cargo or competing in bidirectional transport, KIF1A detaches from the microtubule much more easily than other motor proteins.
- KIF1A’s ability to quickly reattach to the microtubule and ramp up its movement compensates for easy detachment, like short sprints adding up to a marathon.
Main Takeaways
- KIF1A doesn’t operate in a vacuum! It coexists with other motor proteins that cooperate and compete to get cargo to the right place. This means that KIF1A movement isn’t just about its speed, but the force it generates as well.
- KIF1A is tenacious! It falls off of microtubules often but is very likely to reattach and keep walking.
- Adaptor proteins that subtly change how KIF1A, dynein, or other motor proteins operate could be potential indirect targets for KAND therapies by changing cargo movement.
Q&A
- How might we expect different KIF1A mutations to compete against other motors? Different mutations can impact different aspects of KIF1A biology, including force and speed. When motors pull in opposite directions, an important factor is how strongly they pull (this is called force generation). Slow KIF1A mutants may still generate enough force to compete with other motors, but “weak” KIF1A mutants may have more trouble.
- Can we target competing motors to improve mutant KIF1A’s cargo transport? Inhibiting other motors could help KIF1A’s ability to compete, but all motor proteins perform important functions, and weakening one type to help KIF1A could have dramatic side effects as well. Biological systems rely on a certain amount of balance for the system to function properly, and we want to be care to not swing the balance too far in one direction or the other.
- What are adaptors, and why are they so exciting in the context of KIF1A? Adaptors are a type of protein that can link two proteins together. Adapters can have many different effects on motor proteins – some increase or decrease movement to different degrees, facilitate cargo binding, and can bind at different sites. By learning more about adapters, we can discover new ways to fine-tune KIF1A function, which could be grounded in the challenges that specific KIF1A mutations present.


Very clear and interesting, Thanks!