#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dominique Lessard and Dr. Dylan Verden of KIF1A.ORG summarize newly published KIF1A-related research and highlight progress in rare disease research and therapeutic development.
Goodbye with Gratitude: Dr. Dominique Lessard
It is with heavy hearts that this week we say goodbye to Dr. Dominique Lessard, Chief Science Officer of KIF1A.ORG.
Dr. Lessard became part of the KIF1A.ORG community through her graduate research on the biomechanical properties of KIF1A. After speaking at our 2019 conference and receiving her doctorate, Dr. Lessard joined KIF1A.ORG as its first staff scientist, bringing with her a wealth of expertise in KIF1A, both her own and that of her research colleagues.
Those colleagues would become the core of KIF1A.ORG’s Research Network, which now includes over 140 members. During her tenure, Dr. Lessard created and advanced KIF1A.ORG’s Therapeutic Acceleration Program, facilitating the creation of patient-derived cell lines, mouse models, and drug screening platforms that are the bedrock of our search for treatments.
Throughout this process Dr. Lessard has always put patients at the center of her efforts; her intuitive communication style makes difficult science accessible, and her empathy makes our community’s needs impossible to ignore. We are humbled to have had such an incredible champion spearheading Team Science, and wish her well-deserved success in her next stage.
2023 Conference Speaker Highlights: Dr. Simran Kaur
With our 2023 KAND Family & Scientific Engagement Conference fast approaching, we’re excited to feature some of the phenomenal speakers in our lineup, beginning with Dr. Simran Kaur from the Murdoch Children’s Research Institute in Australia.
Hopefully Dr. Kaur is a familiar face to our community, as she is an integral member of our Research Network who is constantly building collaborations to create patient-centered KIF1A research. KIF1A.ORG provided pilot funding to Dr. Kaur in 2021, which she has leveraged into a 3-year federal grant; she now oversees 5 KAND research projects that utilize our patient-derived cell lines to screen for small molecule therapeutics and test potential gene therapies. She is always fine-tuning her research to best represent our family community, and her enthusiastic commitment is contagious.
We look forward to hearing more from Dr. Kaur in August, but in the meantime you can hear from her and her advisor, Dr. Wendy Gold, in our 2022 interview.
A message from Dr. Kaur:
My research is driven by KIF1A.ORG’s commitment to increase awareness, accelerate therapeutic development and improve the care and quality of children battling with KAND. I am excited to attend the upcoming 2023 KAND Family and Scientific Engagement Conference in person, that will enable our community to reflect upon the progress and to design the next steps to address the KAND therapeutic development challenge collaboratively.
Research Network in Action: Dr. Shi creates KIF1A/KAND-centered undegraduate course at Stanford University
We often stress the value of community in Rare Disease research, because we need to work together to tackle the complicated biology of KIF1A and its effects on health. One of our biggest success stories is the growth of our Research Network to over 140 members. We are always looking for ways to integrate more scientists into our relentless efforts. In our most recent success story, Dr. Rebecca Shi at Stanford, inspired by Research Network members has created an undergraduate course to study KIF1A mutations in worm models. This course wasn’t just about learning KIF1A biology – thanks to collaboration with our very own Dr. Dominique Lessard, Dr. Shi’s students were able to learn about KAND, including the perspectives of families affected by KIF1A mutation. Education is one of the most effective ways of moving our research and medical culture forward, and we’re grateful to Dr. Shi and her students for joining with our community to put patient perspectives at the center of their research.
KIF1A-Related Research
Comparative analysis of two Caenorhabditis elegans kinesins KLP-6 and UNC-104 reveals a common and distinct activation mechanism in kinesin-3
Last week we discussed key differences in cargo transport by different motor proteins, including the difference between the kinesin-3 family (including KIF1A) and other kinesins.
But even among kinesin-3 motor proteins there are key differences in motor function – KIF1A isn’t just like every other protein in its family. These differences allow kinesin-3 motors to perform different tasks in the body. And by investigating these differences, we can get a better idea of what makes KIF1A unique.
In this week’s pre-print* researchers used the C. elegans worm model to compare their version of KIF1A (called UNC-104) to another kinesin-3 motor called KLP-6.
In particular, the authors were interested in how KIF1A and KLP-6 go from inactive to active states. As a refresher, KIF1A and KLP-6 need to form pairs (called dimers) to carry cargo across a microtubule – single proteins fold up in an inactive state, a process called autoinhibition. The question is, what steps need to happen for kinesin-3 proteins to activate, pair up, and start walking?
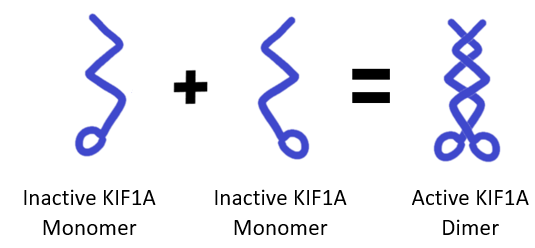
We can imagine two scenarios that would have different implications for KIF1A proteins:
- Kinesin-3 proteins only form dimers when two motors are attached to the same cargo: If KIF1A requires cargo attachment to activate, mutations in the KIF1A’s cargo binding domain (the area where KIF1A attaches to cargo) could prevent KIF1A from activating and walking in the first place.
- Kinesin-3 proteins can form dimers and begin walking independently of cargo: If KIF1A dimers can walk without cargo, mutations in KIF1A’s cargo binding domain could cause traffic jams, with unloaded KIF1A crowding along microtubules and disrupting transport.
To test these possibilities, the authors introduced mutations that released KIF1A and KLP-6 from autoinhibition, meaning these motors could spontaneously form dimers when no cargo was around. They then investigated differences in how UNC-104 (KIF1A) and KLP-6 moved.
This study found that even when KLP-6 is released from autoinhibition, it still doesn’t form dimers or walk along microtubules. It seems that KLP-6 proteins likely form dimers when they’re bound to the same cargo.
In contrast, when UNC-104 (KIF1A) is released from autoinhibition, it begins forming dimers that walk along the microtubules, even when there is no cargo present.
So what’s different between these closely related proteins? The key seems to be a region of kinesin-3 motors called the CC2 (Coiled-coiled 2) domain. The CC2 domain plays a crucial role in helping KIF1A dimerize, and when it’s removed KIF1A can’t walk along microtubules. In contrast, KLP-6’s CC2 domain is much shorter and doesn’t appear to impact dimer formation or transport.
This study supports the idea that when KIF1A is activated, it forms dimers that can then bind to cargo and begin walking, as opposed to KLP-6 motors which only pair up when already attached to cargo.
While most KIF1A mutations occur in the motor domain, we are still actively learning about mutations in other regions, like KIF1A’s stalk and cargo-binding domains. These results provide insight into how these regions play a role in KIF1A regulation, and may give us leads into how they contribute to KAND symptoms.
*What’s a pre-print? Check out this #ScienceSaturday post to learn more
Rare Roundup
Why the underestimated economic burden of rare diseases could be costing the U.S. trillions of dollars
Health is an investment in the future; when we don’t get the care we need early, we tend to require more intense (and expensive) interventions down the road. These costs aren’t just an individual burden, either – schools, workplaces, and public services all see the cost of untreated disorders. And while an individual disease might be rare, collectively over 30 million people suffer from rare diseases in the United States alone. This represents a societal cost of over $7,000,000,000,000 (yes, that’s 7 trillion dollars) per year. Prioritizing rare diseases in research, development, and policy isn’t just a charitable choice; it could be an investment that pays monumental dividends to rare families and our society at large. Read more to learn about the factors involved in incentivizing investment in rare disease treatments.

