#ScienceSaturday posts share relevant and exciting scientific news with the KAND community. This project is a collaboration between KIF1A.ORG’s Research Engagement Team Leader Alejandro Doval, President Kathryn Atchley and Science Communication Director Dr. Dominique Lessard. Send news suggestions to our team at impact@kif1a.org.
Recent KIF1A-Related Research
The fast and superprocessive KIF1A predominately resides in a vulnerable one-head-bound state during its chemomechanical cycle
We are excited to share this hot-off-the-press study out of the Hancock Lab at Penn State University that is focused on the chemomechanical cycle of KIF1A. What is meant by the term “chemomechanical cycle”? This is the process in which KIF1A uses a chemical fuel source (ATP) to produce mechanical action (KIF1A movement). In order for KIF1A to take many steps along a microtubule roadway, this process must occur over and over again in the motor domain of the protein. We often use the analogy of a car engine when referring to the chemomechanical cycle; just like KIF1A, a car needs a chemical fuel source (gasoline) to produce mechanical action (drive).
This study takes a deep look into the intricate mechanics of the KIF1A engine. Specifically, this study reveals that the KIF1A chemomechanical cycle behaves similarly to other kinesin motors (kinesin-1 and kinesin-2 families). However, what is unique to KIF1A is the speed at which it transitions through some of the steps in the chemochemical cycle. Of great importance, this is also one of the first research articles that specifically references KIF1A Associated Neurological Disorder. Thank you to the Hancock Lab for your research and for helping spread the word about KAND to the research world. In case you missed it, here’s a link to one of our favorite articles from the Hancock Lab, characterizing members of the kinesin superfamily as superheroes! If you want to review the basics about KIF1A’s engine, take a look at the video below.
Rare Disease News
One-Time Treatment Generates New Neurons, Eliminates Parkinson’s Disease in Mice
After sharing the news of Ionis Pharmaceuticals’ new KIF1A antisense oligonucleotide (ASO) project, we are always on the look out for reports of ASO therapy success. This article summarizes a recent study from the Fu Lab at the University of California San Diego School of Medicine involving an ASO designed to treat a mouse model of Parkinson’s disease (PD). But first, how does one turn an unaffected mouse into a model for PD? In humans, PD is characterized in part by a lack of dopamine, a powerful chemical messenger in our brains; this lack of dopamine is caused by the death of dopamine-producing neurons in specific parts of the brain. To mimic this, researchers use a chemically induced mouse model of PD in which a chemical that kills dopamine producing neurons is applied to the brains of mice.
It is important to note that this mouse model does not recapitulate all symptoms of PD, but is a good tool to study pathologies caused by a loss of dopamine. The ASO used in this study targeted a protein known to control gene expression called PTB. When the translation of the PTB protein was inhibited by this ASO, what they found was surprising; non-neuron cell types in the mouse brain started to turn into neurons! Because of this, new neurons were able to produce dopamine and dopamine levels returned to almost normal levels. Furthermore, the motor function of these mice drastically improved. While it is important to remember that a mouse is not a human and this mouse model does not recapitulate all aspects of PD, this study is a fantastic proof of concept for ASO PD treatment.
“It’s my dream to see this through to clinical trials, to test this approach as a treatment for Parkinson’s disease, but also many other diseases where neurons are lost, such as Alzheimer’s and Huntington’s diseases and stroke… And dreaming even bigger — what if we could target PTB to correct defects in other parts of the brain, to treat things like inherited brain defects?… I intend to spend the rest of my career answering these questions.”
Dr. Xiang-Dong Fu
Lifting weights makes your nervous system stronger, too
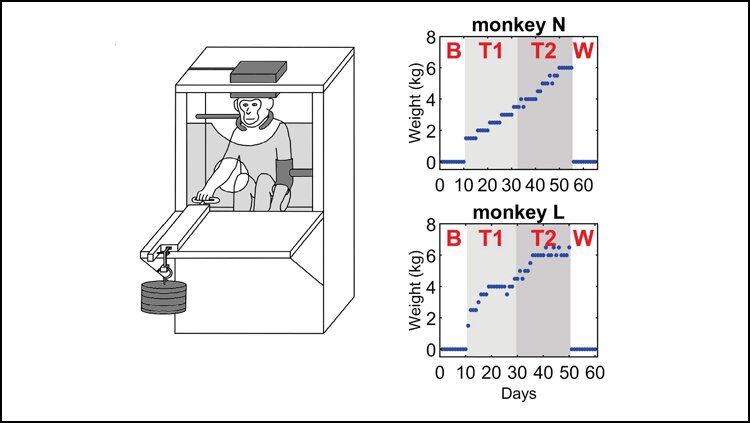
While you are probably aware that lifting weights is a great way to make your muscles stronger, did you know that weight training can make your nervous system stronger too? This article summarizes a recent study that put monkeys through a 12-week weight training program focused on only one arm. Along with weight training, the researchers of this study measured the strength of electrical signaling in the nervous system every day. What they found was that the nervous system electrical signals were stronger on the side of the weight trained arm, as opposed to the arm that did not go through weight training.
This study helps us learn more about a part of our body known as the neuromuscular network. Our neuromuscular network is mainly comprised of our muscles and the nerves that connect to muscle fibers, known as motor neurons. When a motor neuron sends an electrical signal, our muscles are able to contract and generate movement in a voluntary (like flexing your bicep) or involuntary manner (like a heart beat). Specifically, this study reveals that weight training adaptations of our nervous system occur in the reticulospinal networks of our neuromuscular network, which is involved in locomotion and postural control.
A Gene-Editing Shot Could Protect Against Heart Attacks
According to the World Health Organization, cardiovascular disease (CVD) is the leading cause of death globally. Moreover, the risk of developing CVD has been linked to one’s cholesterol levels, which can vary depending on genetic background and environmental factors. This article summarizes advances in a new question being asked by scientists: could this risk be lowered by using gene editing technology? The biotech company Verve Therapeutics has begun tackling this question in monkeys by targeting two genes that regulate “bad” LDL cholesterol. While still in the early stages of development, a significant drop in the monkeys’ LDL cholesterol was observed within two weeks with no notable off-target effects. This presents a new and novel approach to treating CVD and Verve Therapeutics is hoping to begin clinical trials by 2023.

