#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dylan Verden of KIF1A.ORG summarizes newly published KIF1A-related research and highlights progress in rare disease research and therapeutic development.
KIF1A-Related Research
Genetic overlap between ALS and other neurodegenerative or neuromuscular disorders
How do you define a disease?
Traditionally, diseases were defined by their symptoms. If a physician sees a patient with symptoms X, Y, and Z, they diagnose them with a disorder associated with X, Y, and Z. Hopefully the intersection of symptoms let them pinpoint a specific or rare diagnosis, but sometimes multiple disorders share X, Y, and Z.
In recent decades, genetic testing has given another way to define many diseases; knowing that X, Y, and Z can be caused by a gene K mutation reinforced the bridge between symptoms and biological causes, informing disease research and treatment.
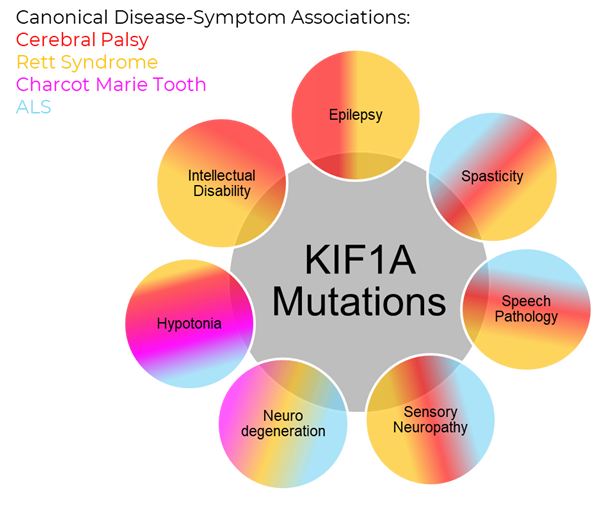
Combining these two styles of diagnoses has led to the realization that many common symptom-defined disorders may be umbrellas that contain multiple genetic disorders. But as we’ve seen in KAND, the same gene mutation may cause different symptoms as well; so we’re left with a complex web where KIF1A mutations are represented across diverse disease communities.
The Study
In this week’s article, researchers in Norway investigated gene mutations in patients with Amyotrophic Lateral Sclerosis (ALS), a neurodegenerative disorder that compromises motor neurons responsible for controlling voluntary muscle movement. There are estimated to be 30,000 people with ALS in the United States alone, only a small portion of which have an associated genetic mutation.
In this study, 279 ALS patients and their physicians in Norway provided information about their clinical history, and had their genome assessed by Exome Sequencing, with a particular focus on 510 genes associated with neuromuscular and neurodegenerative disorders, including KIF1A. After finding mutations in patients, they filtered potential ALS interactions with the following criteria:
- Do the gene’s mutation-associated symptoms match ALS?
- Has the variant been reported as pathogenic?
- Is the variant in the gene predicted to the pathogenic?
- Is the variant seen in 2 or more ALS cases?
Cases matching these criteria were then included in a “Variant Table” of ALS-associated genetic mutations. This led to the identification of 25 patients (~9% of total) carrying one of 20 potential variants in “genes causing ataxia, HSP, PD, mitochondrial disorders, sensory neuropathy, spinal muscular atrophy (SMA), and Charcot–Marie–Tooth disease (CMT), and in genes known to increase ALS risk.”
3 of these 25 patients had KIF1A mutations: V178M, A619G, and R711W. Notably, the V178M mutation is in KIF1A’s motor domain, while A619G and R711W are located in KIF1A’s coil-coil domains, indicating that multiple mechanisms of KIF1A dysfunction could contribute to ALS phenotypes. The V178M and R711W mutations were also characterized as Variants of Uncertain Significance (VUS) in genetic databases, highlighting an ongoing gap between the identification of genetic mutations and the disease diagnosis.
Other genes with potential links to KIF1A were also identified: LRRK2 is a Parkinson’s Disease-associated regulator of KIF1A cargo binding; and NEFH‘s C. elegans counterpart may stabilize KIF1A transport along microtubules.
When talking about all the different types of diagnoses KAND families can receive, I’m often asked if that means “X is actually KAND?” The answer is more complicated; of the 279 ALS patients in this study, only 3 had KIF1A mutations, and 19 other genes were identified. But this points to the importance of genetic testing in more common disorders.
As symptom-based and genetic-based diagnostic systems merge, it’s natural for things to get a bit messy; curing causes and treating symptoms are both important approaches, and systems like insurance are still grounded in symptom-based diagnoses for treatment coverage. Hybrid genetic-symptom diagnoses like KAND-ALS may help reflect the heterogeneity of both groups and help connect patients with similar symptoms and experiences.
Rare Roundup
‘We had no hope’: Patients, advocates testify at U.S. Senate hearing in support of changing FDA rules for rare disease treatments
Overlapping diagnoses highlight the need for collective action by rare disease communities. This week the U.S. Senate had hearings related to the Promising Pathway Act, legislation that aims to accelerate access to new treatments for patients with rare diseases. Advocates included patients with a wide array of disorders, including ALS, unified by a common cause: accelerating the approval process for promising drugs so they can help patients sooner. As our Research Network continues their progress searching for treatments and cures for KAND, it’s important that we pave the road for those opportunities. Stay tuned for opportunities to help KIF1A.ORG advocate for our community and others like ours.
Boston Hockey Tournament For Rare Disease
KAND patients are warriors, so what better place to raise money for KAND research than the Warrior Ice Arena in Boston, Massachusetts? Today, hockey fans and community supporters will gather to watch Boston’s first responders face off in a charitable hockey tournament! Proceeds will go to the KIF1A Rare Pediatric Disease Program at Boston Children’s Hospital, led by Dr. Wendy Chung.