#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dominique Lessard and Dr. Dylan Verden of KIF1A.ORG summarize newly published KIF1A-related research and highlight progress in rare disease research and therapeutic development.
KIF1A-Related Research
Pre-print: The net charge of the K-loop regulates KIF1A superprocessivity by enhancing microtubule affinity in the one-head-bound state
Unlike vehicles on a road, KIF1A molecules aren’t held to a surface by gravity – proteins diffuse through the cell’s cytoplasm, and KIF1A that detaches from the microtubule can float away. But KIF1A is known for moving long distances, so how does it stay on track? In this week’s article, researchers from the Hancock lab studied how a region of KIF1A called the K-loop acts as an electrical tether between the motor protein and the microtubule.
The K-loop is a region of KIF1A that is more positively charged than similar regions in other kinesins like Kinesin-1. So the authors created KIF1A with a kinesin-1 loop, and kinesin-1 with a KIF1A loop. They found that removing KIF1A’s positively charged K-loop severely reduced the distance it could travel across a microtubule.
Because the K-loop is positively charged and the microtubule is positively charged, one possibility was that the K-loop acts like a magnet, tethering KIF1A to the microtubule between steps so it doesn’t float away. To test this, the authors modified Kif1A’s K-loop, either increasing or decreasing its positive charge – decreasing charge drastically reduced KIF1A’s ability to move along the microtubule.
A short travel distance could happen for multiple reasons – it might be that the motor simply never latches onto the microtubule, or it could be that the motor falls off and floats away after fewer steps. By investigating these components separately, the authors found that swapping out KIF1A’s K-loop causes KIF1A to fall off the microtubule between steps when it is weakly attached (see below).
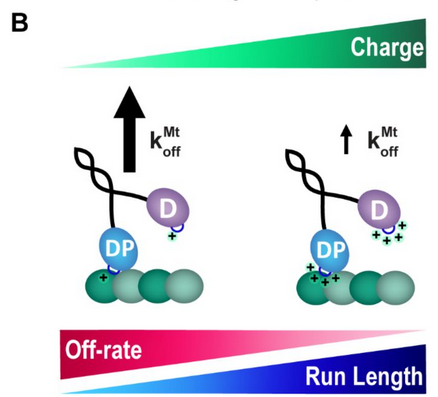
One of the reasons KAND is so heterogeneous is that mutations can have different impacts on KIF1A. There are several human KAND mutations that occur near the K-loop, including P305L and R307Q, which similarly show reduced travel distance. Investigating how the location and nature of a mutation changes KIF1A function is crucial for understanding the disease and developing treatments.
*What’s a preprint? Check out this #ScienceSaturday post to learn more!
Rare Roundup
Lab grown ‘mini eyes’ unlock understanding of blindness in rare genetic condition
In disease research, the model makes all the difference. Molecular, cellular, and animal models all have benefits and drawbacks that allow us to understand a disease, and test potential therapeutics. A challenge for cellular models can be that cells in a dish don’t necessarily assemble as they would in tissue. This is one reason organoids are gaining traction in disease modeling. Organoids are cell cultures that have been primed to grow and connect in a tissue-like manner, more accurately reflecting physiology. Recently, researchers at the University College London have developed “mini eyes” from patient-derived cells to study blindness in Usher syndrome, a rare genetic disorder. The researchers were able to determine which cell types were dysfunctional in these mini eyes, and which gene pathways were being inappropriately activated due to Usher mutations. Down the line, this system may be used to test compounds to treat Usher syndrome.
Studying disorders with multiple models is the best way to make sure findings are robust and generalizable. We’re excited to learn more about these mini eyes, and grateful to members of our Research Network at MCRI for developing organoid models for KAND.

