#ScienceSaturday posts share relevant and exciting scientific news with the KAND community. This project is a collaboration between KIF1A.ORG’s Research Engagement Team Leader Alejandro Doval, President Kathryn Atchley and Science Communication Director Dr. Dominique Lessard. Send news suggestions to our team at impact@kif1a.org.
Recent KIF1A-Related Research
Overexpression of Kif1A in the Developing Drosophila Heart Causes Valvar and Contractility Defects: Implications for Human Congenital Heart Disease
While the vast majority of KIF1A protein is found in our nervous system, this is not the only place we can find KIF1A in the human body. In fact, there are low levels of KIF1A expression in many places in our bodies, including our heart! This paper is investigating the role of KIF1A in left-sided heart defects resulting from an alteration of the KIF1A gene known as balanced chromosomal translocation. While still altering the genetic code of KIF1A, a balanced chromosomal translocation is different than a KAND-causing mutation. A chromosomal translocation occurs when one part of a chromosome (a cellular structure that contains our DNA) breaks off and is swapped with or attached to another chromosome. When a balanced translocation occurs, this means that there is no gain or loss of genetic information. While the amount of genetic information may stay the same, the location in which this breakage occurs can have detrimental effects. In the case of this paper this breakage occurred in the KIF1A gene, leading to changes in KIF1A protein expression. Because this patient also had left-sided heart and aortic valve defects, this lead researchers to investigate the impact of KIF1A protein expression on heart function. Most notably, they found that KIF1A over expression, or too much KIF1A, leads to structural changes in the heart.
Association of microtubules and axonal RNA transferred from myelinating Schwann cells in rat sciatic nerve
Schwann cells play an important role in the neuronal health of our peripheral nervous system, or our nervous system outside of the brain and spinal cord. To do so, Schwann cells wrap around the axons of neurons and produce myelin, the insulating material that helps increase the strength of electrical signals between neurons. This paper is investigating the cross-talk between Schwann cells and neurons, specifically investigating how RNA, a type of genetic material, is exchanged between these two cell types. This exchange relies on the trafficking of RNA molecules along axonal microtubules, mediated in part by kinesin motors. Due to the high levels of KIF1A expression in neurons, the authors investigated if KIF1A was the motor responsible for this process. Surprisingly, the answer was no! Instead a close relative of KIF1A, a kinesin known as KIF1B, was shown to facilitate this process along with another kinesin, KIF5B. From this we learn that, while KIF1A is involved in a lot of processes within our neurons, it’s not involved in all of them.
Rare Disease News
Promise to restore hearing
Many of our recent Rare Disease News articles have focused on how gene therapy is being used to correct a multitude of different disease- or disorder-causing gene mutations. It is safe to say that gene therapy, specifically base editing, is being investigated on a broad and comprehensive scale to address the treatment of rare diseases. This article provides an example of how gene therapy researchers are working to correct recessive mutations of different genes responsible for genetic hearing loss. Give this article a read to learn more about the challenges and solutions researchers have navigated in trying to fix this problem.
“We’re actually going after quite a few genetic diseases now, including some prominent ones that have caused a lot of suffering and energized pretty passionate communities of patients and patient families to do anything to find a treatment.”
Researchers identify two marine molecules with therapy potential against Alzheimer’s disease
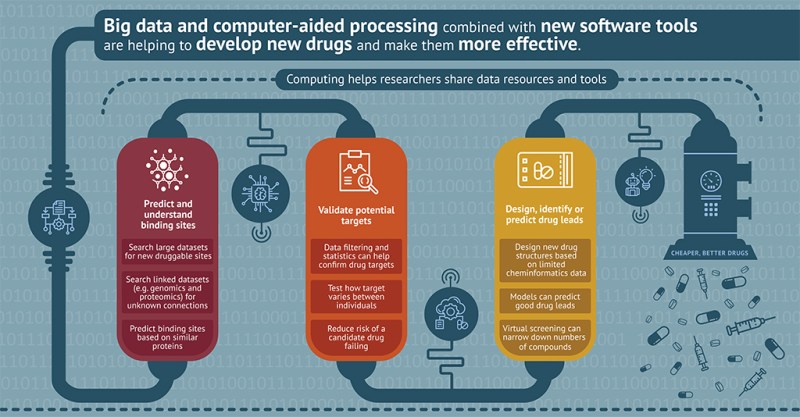
In last week’s Science Saturday, we discussed the concept of “neuroplasticity,” or the brain’s ability to reorganize and form new connections between neural circuits throughout an individual’s life. This week we are featuring an article that connects positive changes in neuroplasticity to two new drug compounds aimed at treating Alzheimer’s disease. These compounds target a protein called GSK3B, a well-known and targeted protein in Alzheimer’s disease drug discovery. Part of this study researched potential compounds at the “in silico” level of investigation, meaning “performed on a computer or computer simulation.” In other words, in silico research uses powerful computational tools to address biological questions. Take a look at the infographic below, detailing how computers are helping with drug discovery!