#ScienceSaturday posts share relevant and exciting scientific news with the KAND community. This project is a collaboration between KIF1A.ORG’s Research Engagement Team Leader Alejandro Doval, President Kathryn Atchley, Science Communication Associate Aileen Lam and Chief Science Officer Dr. Dominique Lessard. Send news suggestions to our team at impact@kif1a.org.
This week we have a very special edition of #ScienceSaturday that highlights a handful of recent KIF1A.ORG advancements!
New Resource From KIF1A.ORG
Research Simplified with Aileen Lam and Dr. Richard J. McKenney

This week, KIF1A.ORG published a NEW Research Simplified article created by Aileen Lam and Dr. Richard J. McKenney from the McKenney Lab at the University of California, Davis. In this resource, Aileen and Dr. McKenney walk us through their recent publication investigating a specific structural component of KIF1A and how disease-causing changes in this location lead to KIF1A dysfunction. Thank you Dr. McKenney for being a committed member of our Research Network! An enormous thank you to Aileen Lam who not only has a history of being an excellent KIF1A researcher, but also has spent the last 9 months as KIF1A.ORG’s Science Communication Associate. Please help us share thanks and best wishes to Aileen as she leaves this position to start medical school at Western University of Health Sciences!
Newly Published KAND Research
De novo disease-associated mutations in KIF1A dominant negatively inhibit axonal transport of synaptic vesicle precursors
Great news coming from our Research Network this week! Dr. Shinsuke Niwa and his team recently uploaded a preprint manuscript of their work regarding KIF1A and KAND. Their study focused on establishing a KAND model in C. elegans, or worms, via the CRISPR/Cas9 system that will be useful in analyzing the molecular biology of the disease in vivo (in a living organism). Additionally, these researchers looked into the behavior of disease-associated KIF1A motors and how they would affect wild-type (i.e. non-mutated, or “healthy”) KIF1A motors when forming heterodimers.
Quick Explanation from KIF1A.ORG
Before going further, let’s take a look at an illustration created by Kat Atchley to help demonstrate this concept. Keep in mind that KIF1A proteins usually work in pairs, in a process that scientists call “dimerization.” When a single KIF1A protein is on it’s own, it’s called a monomer. When it combines with another KIF1A protein, it’s called a dimer.
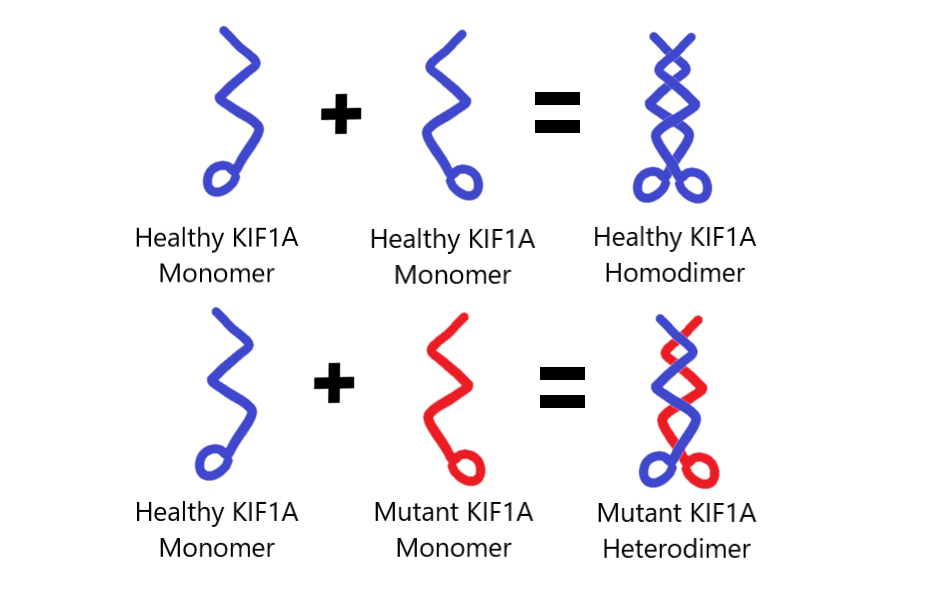
Usually, functioning KIF1A motors are homodimers and are composed of two wild-type (i.e. “healthy”) KIF1A molecules. The heterodimer that these researchers are studying here are composed of a wild-type KIF1A molecule and a mutant KIF1A molecule. In other words, one mutated and one non-mutated KIF1A motor are tethered together and trying working as a team, which we suspect is happening in a disease state system. By conducting these experiments, Niwa Lab hopes to gain further insight on the inhibition of mutant KIF1A on wild-type KIF1A.
Now, back to Dr. Niwa’s paper…
From their findings discussed in the manuscript, Dr. Niwa and his lab showed evidence that KIF1A disease mutants significantly impair the motility (i.e. the ability to move) of wild-type KIF1A when KIF1A heterodimers are formed, which then inhibits axonal transport as a whole. Their investigations show that disease-associated KIF1A disrupts motility and that the wild-type/mutant heterodimers have reduced motor properties. The observation that disease mutants strongly affect wild-type KIF1A when heterodimers are formed also suggests that certain mutants behave to inhibit wild-type KIF1A in a dominant negative fashion, meaning that one mutant KIF1A molecule in the heterodimer can drastically inhibit transport and cause disease. Through this research, they hope to provide more insight on the effects of mutant KIF1A and contribute a foundational worm model that can be used for drug screenings to find future KAND therapies. To read more about the Niwa Lab’s research findings, check out the article below. Thank you Niwa Lab for being a committed member of KIF1A.ORG’s Research Network!
Recent KIF1A-Related Research
Specific KIF1A–adaptor interactions control selective cargo recognition
With great complexity comes great responsibility and regulation. As KIF1A is an intricate motor protein that plays many important roles in brain function and development, high levels of control and management are necessary for this motor to carry out its performance correctly. Since there are so many proteins and mechanisms that are involved in the regulation of KIF1A, researchers are continuously working to uncover the multiple components that participate in these processes.
In this paper, scientists dive into identifying the KIF1A domains, or structural components, that contribute to the function of KIF1A motor activity. As the KIF1A protein is composed of different parts that participate in different roles, these researchers were able to show which domains were responsible for autoinhibition, motor dimerization, and cargo binding. For a little recap, autoinhibition is important because it prevents the use of excess energy when KIF1A doesn’t need to be functioning by putting the motor in an inactive state. Motor dimerization helps bring two KIF1A monomers together to then allow the motor to walk along the microtubules tracks. Lastly, cargo binding is essential for the transportation of nutrients, signals, and organelles throughout the cell. In addition to dissecting the roles of the KIF1A domains, these researchers also determined that phosphorylation, or the process of adding a phosphate group to a protein, is crucial for the motor to bind to vesicles that transport cargo. Furthermore, they identified that specific KIF1A adaptors, or proteins that can interact with KIF1A to regulate its function, are responsible for directing cargo to their specific locations within the cells. Altogether, this research demonstrates that these forms of regulation contribute to the selective KIF1A cargo trafficking that is so essential to proper brain function and development. To read more about these instrumental findings, check out the article below!
Bioinformatics Analysis of KIF1A Expression and Gene Regulation Network in Ovarian Carcinoma
A majority of the time, we associate KIF1A with the brain and neurological disorders, but did you know that KIF1A could potentially be a marker for ovarian cancer (OC)?
Recently, researchers showed in a study that KIF1A has a potential role in cancer pathways. Due to the high expression levels of KIF1A protein observed in OC tissues than in normal tissues, scientists believe that KIF1A could be a possible biomarker for OC, as they saw a correlation between KIF1A and poor clinical characteristics of OC. As a quick reminder, a biomarker is an indicator that is measurable and can be used to detect the severity or presence of a disease. In this case, determining a biomarker for OC would be extremely valuable for the diagnosis process for patients that may have this disease since it would help them receive more timely and appropriate care and treatment. These researchers conducted this study to determine if there is any clinical significance of KIF1A expression levels in OC, especially in the development of the disease. By using the Cancer Genome Atlas (TCGA) OC data, researchers compared expression levels of KIF1A in OC tissue with that in normal tissue and concluded that KIF1A was highly elevated in OC tissues. From these findings, they believe that KIF1A can be a promising biomarker for OC that can significantly help patients with the prognosis of this disease. Curious to learn more about KIF1A’s correlation with OC? Check out the article below!
KIF1A.ORG In The Spotlight
Teaching Friendship and Inclusion on KIF1A Day
More than awareness: learn how KIF1A Day sparked friendship and inclusion at one elementary school, thanks to a special Speech-Language Pathologist, Arica Altobello.
“The group of kids were imitating Sadie’s play to engage with her and they explained to Sarah that, even though Sadie is blind, they know how to say ‘hi’ to her. Sadie’s recess times at school are now filled with peers of all ages hopping alongside her in the sand …”
Arica Altobello, Speech-Language Pathologist, caregiver and friend to KIF1A superhero Sadie

