#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dominique Lessard and Dr. Dylan Verden of KIF1A.ORG summarize newly published KIF1A-related research and highlight progress in rare disease research and therapeutic development.
KIF1A-Related Research
De novo mutations in KIF1A-associated neuronal disorder (KAND) dominant-negatively inhibit motor activity and axonal transport of synaptic vesicle precursors
We all have two copies of each gene, one from each parent. So why is a single copy of mutant KIF1A enough to cause KAND even when patients have a healthy copy?
When a mutation impacts one copy of a gene but not the other, it is called a heterozygous mutation (from hetero: different, and zygous: referring to a fertilized egg). When a heterozygous mutation causes disease it is called a dominant mutation, because it overrides the healthy gene.
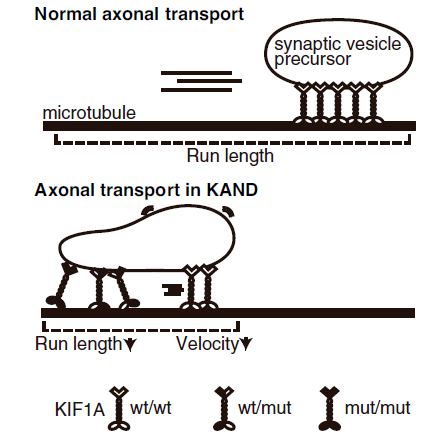
In this week’s paper, Dr. Shinsuke Niwa’s group at Tohoku University investigated the impact of heterozygous mutations on KIF1A function in the C. elegans worm model. C. elegans are easy to genetically manipulate, and their transparent bodies allow researchers to see neuronal proteins move in real time. The worm version of KIF1A is called unc-104.
The group introduced heterozygous mutations to C. elegans that match the KAND mutations R11Q, R254Q, and P305L. These worms had reduced movement, and KIF1A cargo ended up in the wrong places within neurons. The findings suggest that when mutant KIF1A and healthy KIF1A cooperate to carry a cargo, the mutant KIF1A holds healthy KIF1A back. KIF1A movement deficits depended on the specific mutation, which could inform differences in KAND symptoms across our community.
The researchers also performed a “suppressor screen”: worms carrying KAND mutations are given additional random mutations. When a random mutation helps the worms move normally again, that mutation is said to “suppress” the KAND mutation. This second mutation restored KIF1A movement by changing the protein’s charge and increasing its ability to bind to the microtubule. While we wouldn’t want to treat KAND by introducing more mutations, screens like this teach us about KAND mechanisms and may inform future drug targets to improve KIF1A function.
We’re grateful to Dr. Niwa’s group for being active members of the KIF1A Research Network and continuing to leverage basic science to accelerate our understanding of KAND and potential therapeutics.
Rare Roundup
Medicare could have saved $3 billion buying drugs the Mark Cuban way
As KIF1A.ORG and the Research Network continue our #relentless search for KAND therapeutics, it’s important to acknowledge that the journey doesn’t end with finding a drug that works; these medications are often a continuous need, and purchasing them can be its own challenge. In the US, at least 18 million people can’t afford the medications they’ve been prescribed due to high prices or insurance incompatibility. In many cases, Medicare purchases drugs at a price that’s inflated by intermediate steps, which ultimately costs patients more. In the absence of institutional reform, people and groups are developing other solutions. For example, Governor Newsom recently announced that California will begin producing its own insulin to sell near at cost to residents.
In the private sector, Mark Cuban launched Cost Plus Drugs, which purchases generic medications from manufacturers and sells them at a small markup. While this service doesn’t accept insurance, out-of-pocket costs can still be lower than co-pays for some medications. A recent study found that if Medicare had been able to purchase 77 medications at Cost Plus Drug prices in 2020, it would have saved the government $3 billion.
These solutions that bypass the traditional pharmaceutical and insurance market speak to the need for integrated support for patients whose health and quality of life rely on medication.
Designing a tool for better diagnosis of rare and genetic diseases
As our collective understanding of disease increases, the process of diagnosis by an individual physician can be challenging. Clinicians must wade through a complex network of overlapping symptoms and correlate it with genetic or acquired causes to identify a disease and potential treatments. It’s no wonder that many of the patients with one of 10,000 known rare diseases, including KAND families, are misdiagnosed as having other disorders.
To help clinicians narrow down their diagnoses, in 2017 a team of researchers developed PubCaseFinder, an online tool that allows doctors to input patient symptoms and compare them to a large database of diseases and their causative genes. PubCaseFinder was recently updated with a more robust interface and will be better equipped to identify rare and genetic disorders. Creating these kinds of tools plays an invaluable role in equipping clinicians to take advantage of advances in medical research.

