#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dominique Lessard and Dr. Dylan Verden of KIF1A.ORG summarize newly published KIF1A-related research and highlight progress in rare disease research and therapeutic development.
KIF1A-Related Research
Kinesin-1, -2 and -3 motors use family-specific mechanochemical strategies to effectively compete with dynein during bidirectional transport
When we talk about KIF1A mutations changing cargo movement, it isn’t as simple as kinesin moving faster or slower. This is partly because microtubules aren’t just empty roads in the cell, and KIF1A isn’t the only kind of vehicle: Cargo might be attached to multiple kinds of motor proteins that move in different directions.
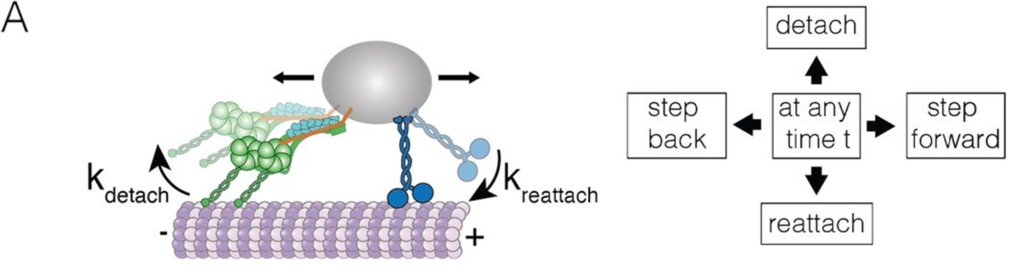
Dyneins are motor proteins that engage in this competition. While kinesins carry cargo away from the cell body (the “plus” side of the microtubule), dyneins carry cargo toward the cell body (the “minus end”). However, many questions remain about how competing motor proteins decide which direction to take a cargo.
In this week’s pre-print* article, Research Network member Will Hancock and colleagues investigated how different kinesins compete with dynein to transport their cargo.
To test this, the researchers tethered single kinesin and dynein complexes with a “rope” of DNA and tracked their movement along microtubules. They found three patterns of movement for kinesin-dynein pairs.
- During paused periods, the dynein anchored itself in an inactive state and couldn’t be moved by the kinesins.
- During fast periods, dynein-tethered kinesins moved toward the plus end almost as fast as untethered kinesin, possibly because dynein was only loosely attached to the microtubule.
- During slow periods, the dynein and kinesin engaged in a tug-of-war that resulted in very slow movement.
Because we know that KIF1A transports cargo quickly in cells, it seems possible that KIF1A and dynein stop their tug-of-war during active transport of cargo. Learning what causes dynein or kinesins to detach or reattach to the microtubule might provide us with ideas for new therapeutics. These factors could be diffused in certain parts of the cell, or sections of the microtubule itself.
These findings also raise an important consideration: When we change how KIF1A operates, we may also be impacting dynein-dependent transport, which could have its own effects on cellular health. Understanding KIF1A as part of a cellular ecosystem is important for creating the best possible treatments.
*What’s a preprint? Check out this #ScienceSaturday post to find out.
Rare Roundup
Sad news from Novartis: dosing suspended in VIBRANT-HD trial of branaplam
Therapeutic development is a difficult process that has many steps. Treatments must be tested again and again across different contexts to evaluate their effectiveness, and just as importantly, their safety.
Branaplam is a genetic treatment originally developed to treat Spinal Muscular Atrophy by rearranging the RNA of a mutated gene (called splicing). This mechanism also showed some promise for another disorder: Huntington’s Disease. With its rapid and severe onset in adulthood, Huntington’s is a neurodegenerative disease with a desperate need for treatments. There was promising evidence for branaplam in animal models of Huntington’s, and initial trials indicated it was safe for healthy adults. Clinical trials in 25 Huntington’s patients began at the top of 2022; this initial phase utilized low doses of branaplam, with plans for higher doses in subsequent groups.
Unfortunately, early evidence collected by an independent monitoring committee suggested that branaplam might be damaging neurons in patients’ limbs, leading to a suspension of the clinical trial. This was a difficult development, both for early participants in the trial and the hopes for branaplam as a Huntington’s treatment. However, catching these potential safety concerns early highlights the role of meticulous oversight in drug development and repurposing. It’s a lesson we take to heart as we continue our relentless search for KAND treatments.


hola, soy Alexandra escribo desde Ecuador, pais de Latino América.
Soy madre de una niña de 6 años, por medio de un examen genético, le diagnosticaron síndrome de NESCAV. KIF1A, Es el primer diagnostico de Ecuador, por favor requiero información de c.946C)T p.R316w. Con la finalidad de dar el mejor cuidado a mi hija.
Un cordial saludo.