#ScienceSaturday posts share relevant and exciting scientific news with the KAND community. This project is a collaboration between KIF1A.ORG’s Research Engagement Team Leader Alejandro Doval, President Kathryn Atchley and Science Communication Director Dr. Dominique Lessard. Send news suggestions to our team at impact@kif1a.org.
Recent KIF1A-Related Research
Enhancing WNT Signaling Restores Cortical Neuronal Spine Maturation and Synaptogenesis in Tbr1 Mutants
This new research article sheds important light on how KIF1A is regulated by a transcription factor associated with autism spectrum disorder, known as Tbr1. The human genome contains ~20,000 genes. However, not all of these genes are actively turned on at the same time throughout our lives. A transcription factor is a protein that is responsible for turning gene expression “on” or “off”; you can think of it like a light switch! Specifically, this article finds that Tbr1 increases KIF1A expression in certain regions of the brain. This article briefly discusses how this finding may relate to KIF1A mutation, but it is important to note that this is a speculative discussion at this point.
Spatial control of membrane traffic in neuronal dendrites
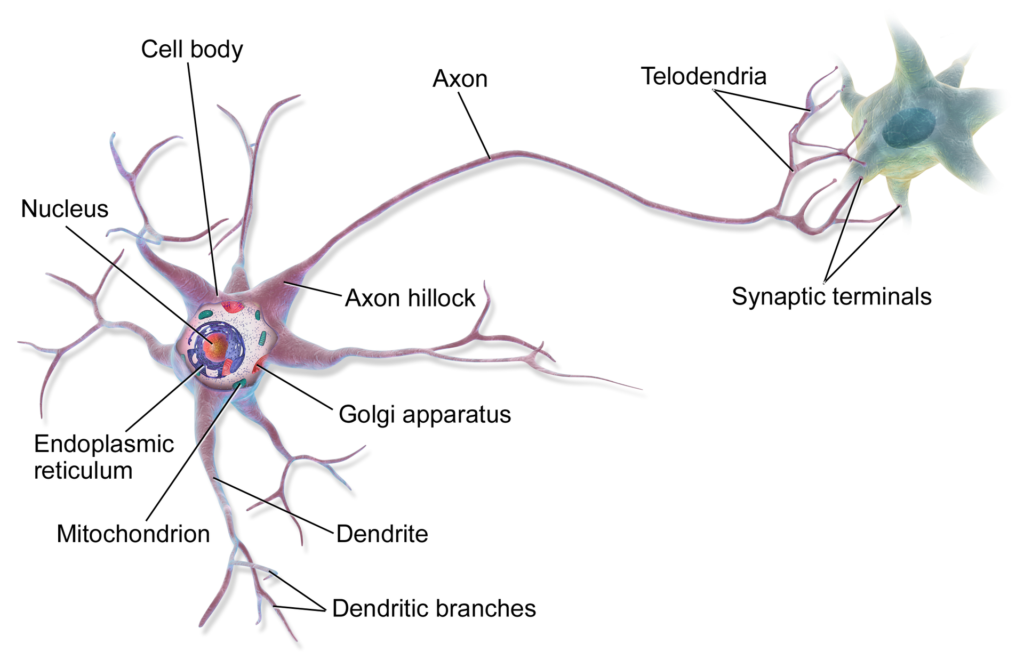
When discussing KIF1A, we often refer to KIF1A cargo transport in a specific neuronal structure known as the axon. However, KIF1A is also known to transport cargo in a part of the neuron known as the dendrites! The dendrites are primarily responsible for receiving signals from other neurons. In order for signals to be received and interpreted effectively, the dendrites must form a complex, “tree-like” geometry. This article comprehensively discusses the unique ways in which molecular motor proteins, including KIF1A, work together to form the complex structure of the dendrites.
Rare Disease News
Study of lipid metabolism in neurons may offer therapeutic possibilities for neurodegenerative diseases
The majority of cargo transported by KIF1A are surrounded by a layer of lipids (fat molecules); these lipids are what KIF1A physically attaches to on the cargo. This article summarizes a study out of the Farese and Walther Lab at Harvard University, focused on how altering the production of a certain type of lipid, called a sphingolipid, leads to changes in the nervous system. This study found that disrupting sphingolipid production leads to an improvement in symptoms of neurodegeneration and increases survival of a neurodegenerative mouse model, hinting at a new therapeutic opportunity. While not directly looking at the effects of KIF1A, this article did find “a link between sphingolipid metabolism and a mutation that affected vesicle trafficking, the process by which molecules are transported to different parts of a cell”.
Brain discovery could have important implications for neurodegenerative diseases
In last week’s Science Saturday, we discussed the idea of repairing a neurodegenerative brain by reprogramming non-neural cell types into neurons. This new article brings up another idea about brain repair: being able to efficiently remove malfunctioning or dead cells. Cell death is a normal part of brain development. Once this has occurred, our brains have mechanisms in place that help clear out dead cells; think of it as the brain’s “clean-up crew”! This article summarizes a recent advancement in our understanding of this “clean-up crew” from the Luken lab at the University of Virginia. Click the image below to learn more!
“Neurodevelopment is a very complicated process… This form of cell death actually plays a role in removing unwanted cells from the brain to establish a healthy [central nervous system] with the correct connections and the right number of cells.”