KIF1A.ORG’s 19th Research Roundtable meeting, “NeuCyte KAND disease modeling drug screening platform,” was presented by Dr. Wayne Poon from NeuCyte. Read on to learn more about how NeuCyte is developing new ways to test therapeutics for KAND.
Attendance
26 RESEARCHERS, CLINICIANS, & BIOTECH REPS

21 RESEARCH INSTITUTIONS & INDUSTRY PARTNERS/ORGS
5 KIF1A.ORG REPS
Who Is Dr. Poon?
Dr. Poon is the Head of Neuroscience at NeuCyte where he works to develop screens for CNS drug discovery. NeuCyte has worked with KIF1A.ORG to generate neurons from patient-derived stem cells. By assessing morphology, axonal transport, and electrophysiological properties in neurons with KIF1A mutations, NeuCyte has created a drug screening platform to test prospective therapeutics. You can get to know more about Dr. Poon here, and see his recent presentation at our 2022 conference here.
Summary
“NeuCyte KAND disease modeling drug screening platform”
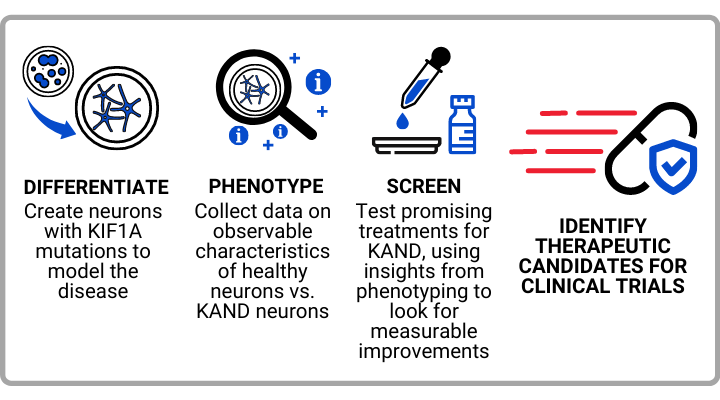
- NeuCyte has created neuronal cultures from two patient-derived cell lines (P305L and E253K). They have been hard at work developing multiple assays (tests that investigate the function of KAND cells), which they are using to inform future drug screening:
- Neuronal outgrowth and morphology: Neuronal growth has a huge impact on brain development and relies on KIF1A movement. P305L cells express less KIF1A in their dendrites and axons relative to their cell body, which means KIF1A isn’t likely transporting cargo in the way it should. When NeuCyte performs its drug screen, it can look for treatments that increase KIF1A in dendrites and axons.
- Proximity Ligation Assay: This assay checks how much KIF1A interacts with tubulin from microtubules. P305L cells had less KIF1A/Tubulin interactions than healthy cells which could compromise KIF1A’s ability to walk along microtubules. Drugs that regulate this interaction could be of therapeutic interest.
- Multi-Electrode Array: This assay tracks the electrical properties of neurons, like hyperexcitability that models seizures. E253K cells were hyperexcitable, which can be a target for drug screening.
- The E253K and P305L mutants behaved differently in these assays, which speaks to the heterogeneity of KAND.
Main Takeaways
- Using patient-derived cells can teach us more about KAND and test multiple potential drugs in parallel.
- Different mutations have different characteristics! A huge strength of NeuCyte’s platform is the ability to customize drug screen assays for each mutation.
- While we’ve started our work with two mutations, our goal is to scale NeuCyte assays up to investigate more mutations in our patient-derived cells at Coriell and genetically engineered cells at Jackson Laboratories. We’re just getting started!
Q&A
- So what now? Now that NeuCyte has developed these assays, we are turning our eyes toward drug screening. KIF1A.ORG and NeuCyte have collaborated to determine a first batch of compounds that will be tested in the NeuCyte platform.
- What happens if a drug seems promising in NeuCyte’s assays? NeuCyte is helping us to narrow our drug search to candidates that make a difference in patient-derived cells. We can take these promising candidates and further assess them in our animals models or in clinical trials to test both efficacy and safety. This is why having a partner who is so engaged with the rest of our Research Network is so important!
Have a compound you think could be screened with NeuCyte’s assays? Let us know by emailing dvlessard@kif1a.org!