Imagine if we could change the fate of KIF1A-Associated Neurological Disorder, a progressive—sometimes fatal—neurodegenerative disorder. Instead of a doctor telling parents there’s nothing they can do to help or save their child, we envision a future where doctors can say “It’s going to be okay. There is treatment.”
This Is How We Fast-Track Studies on Promising Treatments
Even as we continue to develop multiple therapies for KAND with our research partners (such as ASOs, gene-editing, drug repurposing, and structural-based therapies), we recognize the importance of addressing high priority symptoms, such as seizures and spasticity, with small molecule therapies.
With community input on symptoms and goals, our Research Network has identified many potential routes to address KAND dysfunction, with the goal of helping as many KAND patients as possible. But as scientists identify drugs that they think have potential to treat KAND, we can’t just give the drug to our kids first, unless it’s a common drug with a known safety profile. We need to test new compounds in a system that models KAND.

In September 2021, KIF1A.ORG partnered with NeuCyte, a neuroscience research company that specializes in disease modeling, to create a KAND drug screening platform using neurons derived from KAND patients.
Using blood samples donated by patients, NeuCyte has created induced pluripotent stem cells (iPSCs), which can be turned into different cell types like neurons. Now that we have a strong system in place, we can introduce potential drugs.
One reason we chose NeuCyte was their diligence in creating the best possible model of KAND. It’s not just enough to make neurons; the brain has many types of neurons and supporting glial cells, which may play different roles in symptoms like seizures. Creating a co-culture with these different cell types increases our chances of finding the right drugs for KAND. NeuCyte has even created organoids (called mini-brains) that more closely mimic 3D brain structure.
What have we learned?
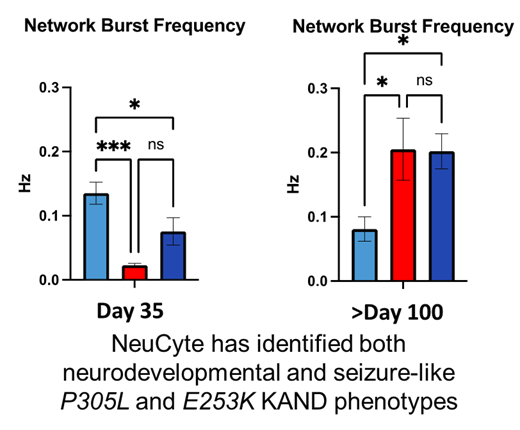
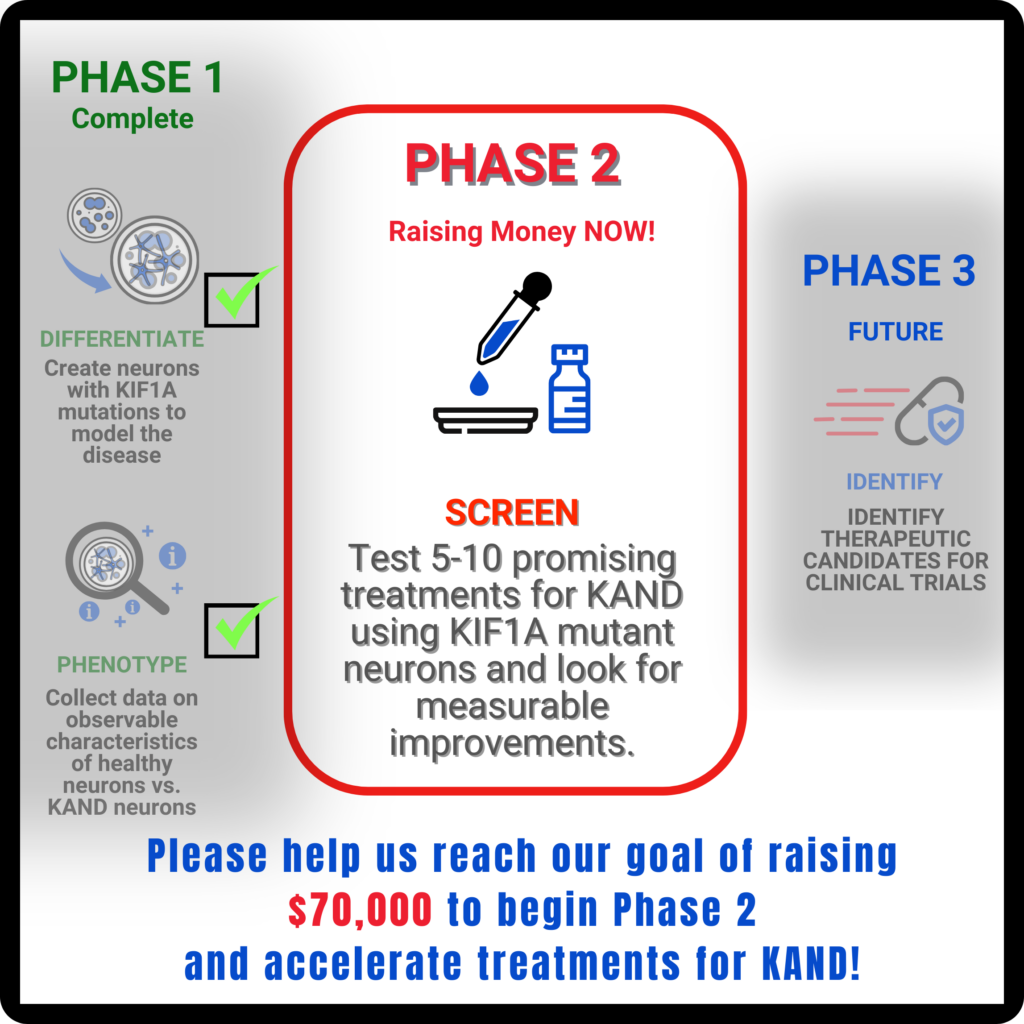
In Phase 1 of our Treatment Accelerator Program (TAP), NeuCyte established that these neurons model KAND symptoms. Early in development, the neurons don’t connect and communicate properly; they also show reduced growth, reflecting developmental symptoms. As the cells develop, the network becomes hyperexcitable, reflecting seizure activity.
We can test drugs by their ability to change network activity both during the early underactive phase, and the later hyperexcitable phase.
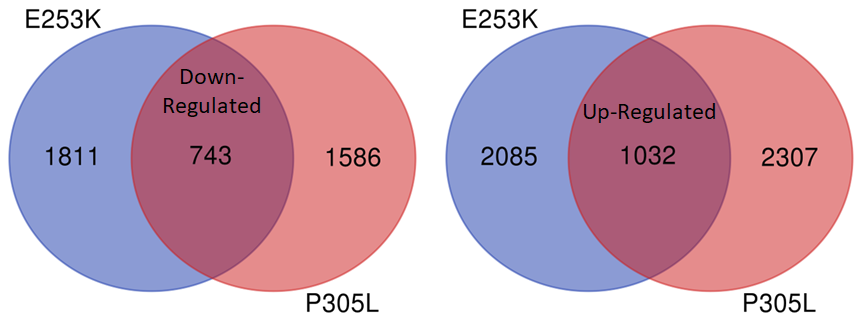
NeuCyte also used RNA Sequencing to look for genes whose expression change in response to mutant KIF1A. This can help us identify promising pathways to compensate for KIF1A mutations. We are now analyzing these gene pathways to look for new potential therapeutics!
What next?
In Phase 2 of TAP we will test a first batch of 5-10 potential KAND therapeutics with antiseizure and neuroprotective properties, and compare them to currently used treatments.
We prioritized therapeutics that have shown promise in other rare diseases with similar symptoms, and therapies that may reduce side effects compared to current options. Our goal is to leverage therapeutics that can be efficiently moved from the bench to the clinic (Phase 3).
Because they don’t target KIF1A mutations directly, the drugs would be more likely to benefit most or all of our patient population, despite the wide range of variants associated with KAND. Before we can determine when the first clinical trial would be, there are still investigations to be completed before we are confident that any treatment we’re exploring should move forward to human trials. Phase 2 will bridge the gap between promising hypotheses and real data for new therapeutics.

You Can Make an Impact
This program will add to our Tools for Development that are freely available to the scientific community, including pharmaceutical and biotechnology companies. These tools require significant time and resources, and are often the reason research institutions and companies choose not to invest in developing treatment for rare diseases. Not with KIF1A. With our accessible tools, we lay the groundwork for fast and efficient therapeutic development.

