#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dominique Lessard and Dr. Dylan Verden of KIF1A.ORG summarize newly published KIF1A-related research and highlight progress in rare disease research and therapeutic development.
News from KIF1A.ORG
Register for the 2023 KAND Family & Scientific Engagement Conference by June 16
We’re pleased to announce that registration for the 2023 KAND Family & Scientific Engagement Conference is LIVE! This will be our first in-person meeting since 2019, and we look forward to convening families and researchers to discuss our path towards treatments for KAND. Registration will close on June 16, so be sure to sign up!
Please note that Conference Registration, KOALA Enrollment, and Hotel Booking are separate forms.
KIF1A-Related Research
Peripheral nerve involvement in hereditary spastic paraplegia characterized by quantitative magnetic resonance neurography
Movement of our bodies is complicated: it requires coordination of signals from multiple brain regions traveling down the spinal cord, and then to our Peripheral Nervous System, where neurons activate and inactivate muscle groups. So when it comes to movement disorders like spasticity in KAND, one of the first questions is: how are different parts of this circuit involved?
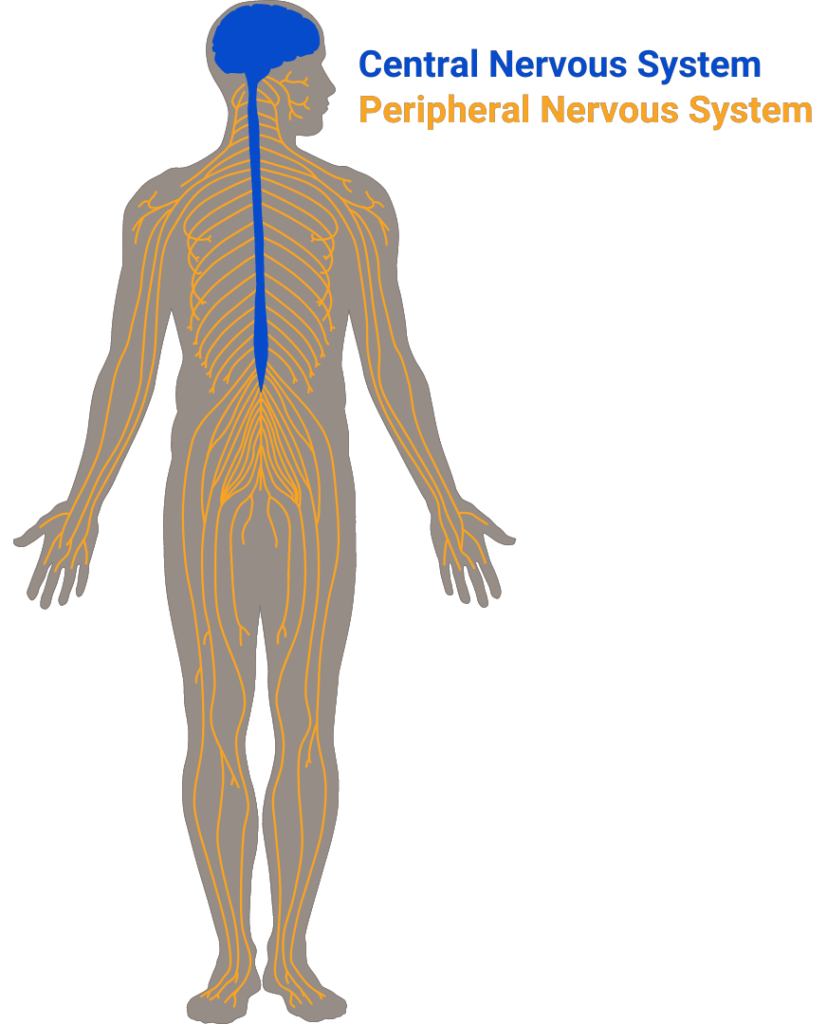
This is an important question because the logistics of treating the central nervous system (your brain and spinal cord) or the peripheral nervous system (your limbs) can differ quite a bit, as we’ve discussed in our KAND and Spasticity post; certain drugs may be better suited for treating central or peripheral symptoms.In this week’s article, researchers utilized a technique called magnetic resonance neurography to investigate the peripheral nervous system of patients with spastic paraplegia (SPG) types 4 and 7. While these are different from the SPG30 that occurs in KAND, their methods of measuring dysfunction of the peripheral nervous system could be useful for many patients with movement disorders.
What is magnetic resonance neurography?
Many in our community might be familiar with Magnetic Resonance Imaging (MRI). This technique uses magnetic fields to pick up on subtle differences between types of soft tissue, providing images of the brain and other organs. This allows physicians to detect tissue loss or dysfunction without harming the patient. For example, atrophy of the cerebellum, a key brain region involved in fine-tuning movement, is commonly observed in MRIs of KAND patients.
Magnetic Resonance Neurography (MRN) is a specific type of MRI that uses the same technology to look at peripheral nerves, like the ones that run from your spinal cord to your leg. Researchers can use MRN to detect the loss of neuronal axons, demyelination, and inflammation, all of which can contribute to spasticity or muscle weakness.
The study
The authors used MRN to look for damage to nerves along the thigh and shin of healthy patients, as well as patients with SPG4 and SPG7. They compared these measurements to the electrical activity in these areas – how well the neurons were able to transmit electrical activity through the leg. They found that patients with SPG4 and SPG7 had signs of axon loss or demyelination, even when electrical activity seemed normal.
This makes sense neurologically: Neurons can take a certain amount of damage and still function relatively well, and healthy neurons can compensate for unhealthy ones. But early damage can lead to electrical dysfunction and neuronal death over time.
Because changes in the nerve structure were detectable even in patients without clear electrical dysfunction, it’s possible that MRN could be used as an early marker for peripheral nerve pathology in patients, better predicting future symptoms in patients with hereditary spastic paraplegias.
However, this study only performed a single evaluation of each patient; it is important to follow up with studies that track MRN signals over time as the disease progresses to understand how to integrate this tool into characterizing spasticity-related disorders like KAND.
Rare Roundup
Novel Genetic Screening Tool Offers Hope for Babies Born With Life-Threatening Metabolic Disorder
This week ABC covered the story of KIF1A superhero Edward Boyer in Australia. Many of the experiences the Boyers discuss will be familiar to our community: Misdiagnosis, progressive symptoms, and the challenges of traveling as a rare disease family. The article also features Dr. Simran Kaur of our Australian research network. We can’t summarize better than the Boyers, so we thank them for sharing and encourage you to read their story.