#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dylan Verden of KIF1A.ORG summarizes newly published KIF1A-related research and highlights progress in rare disease research and therapeutic development.
Meet our Guest Bloggers!
This spring we’ve been quite fortunate to be joined by 6 Master’s students from Columbia University, who have been contributing to our creation of community resources as part of their Seminar in Biotechnology Course. March is Cerebral Palsy Awareness month so today Wangyang, Ada, and Jamie are here to discuss genetic testing in cerebral palsy.
Wangyang Dai
Wangyang is a master student in the M.A. Biotechnology program at Columbia University. She obtained her Bachelor’s degree in Life Sciences from Fudan University in 2023. During her time as an undergraduate student, she conducted research on proline metabolism and its effects on ATF4 regulation. Later, she developed an interest in the RNA world, specifically focusing on alternative splicing and its correlation with neuron cell properties within and across cell types. Now in the Zhang lab, she is involved in a project about analysis of CLIP data using crosslink-induced mutation sites (CIMS) and aims to utilize it as a signature to better understand RBP binding specificity.
Jamie Woych
Jamie received her BS in Biochemistry from SUNY New Paltz in 2018. She previously worked in the Cellular Biology Department at Rutgers University as a research associate, working with zebrafish as models for neural development. She is a driven scientist continuing her studies on the graduate level in biotechnology. She bridges her interests in biology and neuroscience through her work in the Tosches Lab, in the Biological Sciences Department at Columbia University, where she has been the lab manager for the past four years. In the lab, she is currently studying the evolutionary conservation of cell types and circuits involved in the amphibian olfactory system.
Ada Lyu
Ada Lyu is a graduate student completing her master degree at Columbia University. She graduated from UC San Diego with a B.S. degree in human biology in 2023. Her undergraduate research focused on lipid metabolism of NASH at UCSD Medical School and DNA breakage and repair mechanism at Scripps Research. Lyu is currently working in a lab at Columbia University Irving Medical Center studying the influence of cholesterol on cardiovascular health.
KIF1A-related Research
Clinical utility of a genetic diagnosis in individuals with cerebral palsy and related motor disorders
Clinical utility of a genetic diagnosis in individuals with cerebral palsy and related motor disorders
What is Cerebral Palsy?
Cerebral palsy (CP) is a group of non-progressive disorders that affect movement and muscle tone or posture. It is commonly thought to be caused by birth-related factors, which lead to damage or abnormalities in the developing brain, but new work suggests a genetic cause in up to 1 in 4 of cerebral palsy cases.
Cerebral Palsy, KIF1A, and KAND
Several studies reported KIF1A mutations in cerebral palsy cases, and many patients in our community came to us after initial cerebral palsy diagnoses. Similar to cerebral palsy, KAND also involves motor deficits originating in childhood that can lead to challenges with movement, coordination, and balance.
However, there are some key differences. Cerebral palsy results from brain injuries early in development, while KAND arises from mutations in the KIF1A gene that disrupt neuron function. Cerebral palsy leads to lifelong but non-worsening motor issues, whereas KAND often shows progressive decline. This decline could easily be missed over the course of long periods of time. It is important to note that disease progression is, in fact, cause to rule out a CP diagnosis, and a reason to look for other causes like genetic mutations.
These cases have been called cryptogenic CP, reflecting the lack of a known cause, or CP-masquerading conditions, reflecting key differences in symptoms or cause. Recent studies have revealed that a significant proportion of individuals diagnosed with CP may have an underlying monogenic disorder.
KAND may also cause additional symptoms not present in cerebral palsy, including vision loss, and peripheral neuropathy. Cognition is typically spared in cerebral palsy, but KAND frequently also impairs intellectual development.
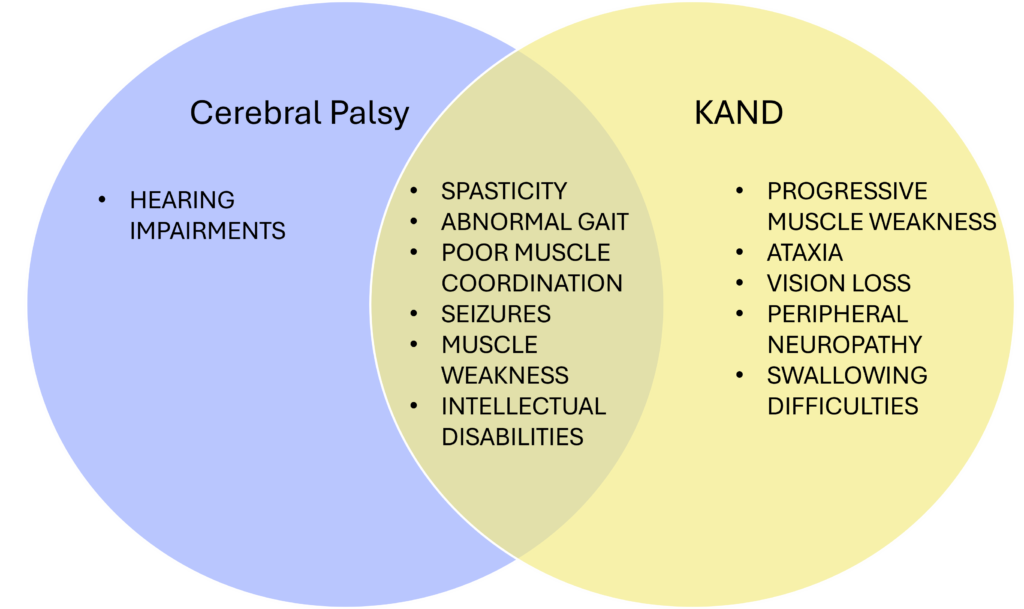
Treatments that are commonly used for cerebral palsy are not always useful or recommended for patients with genetic disorders. Therefore, this distinction is very important for tailored treatment planning, especially for noting that regular monitoring and reassessment of symptoms is necessary.
The Value of Genetic Diagnosis
A genetic diagnosis has a significant impact on the management and treatment of cerebral palsy and related motor disorders in clinical practice. It has been applied to detect genetic variations in the KIF1A gene that are known to cause disease or variations in other genes known to cause symptoms similar to KIF1A-related disorder. It allows for a better understanding of the underlying triggers and processes of these disorders, leading to more personalized clinical management and treatment [1]. Genetic testing can explain the clinical presentations of affected individuals and guide therapeutic interventions based on the genetic diagnosis [2]. In some cases, targeted treatments and preventive interventions can be implemented based on the genetic results, resulting in positive outcomes [3]. Genetic diagnosis also enables better clinical management and targeted genetic counseling for family members at risk of these disorders [4].
The Study
Increased access to genetic testing has improved the identification of genetic causes of cryptogenic CP. However, despite the growing recognition of the contribution of genetics to CP and related motor disorders, the clinical utility of a genetic diagnosis for individuals with CP and CP-masquerading conditions remains limited.
Clinical utility can be defined by the ability of a healthcare intervention to provide meaningful improvements in patient outcomes, quality of life, or prognosis. In terms of this study, this includes actions taken by the group’s healthcare providers following genetic diagnosis. In this paper, the authors aim to characterize the impact of a genetic diagnosis on clinical management and conducted ES on participants with CP and “CP masquerading” conditions.
The authors analyzed data from past patients to evaluate the clinical utility of a genetic diagnosis in individuals with CP and CP-masquerading conditions. The results highlight the clinical utility of a genetic diagnosis in CP and related motor disorders, demonstrating its impact on medication interventions, surveillance actions, and variant-specific actions.
This analysis ultimately resulted in the finding that 93% of individuals included in the study had meaningful changes to their clinical care. A specific example of this impact is described within the theme of patient education. These results report that 43% of the patients involved in the study were either referred to an expert about their specific genetic disorder or counseled about possible prognosis changes. Despite the fact that not all of these genetic mutations are yet fully understood, this points to an amazing amount of intervention and action that can be applied following genetic diagnosis.
It is exciting to see that genetic diagnosis has an impact on clinical outcomes. In my opinion, getting a genetic diagnosis is beneficial to CP patients, or maybe all patients exhibiting certain symptoms with no obvious cause. The genetic diagnosis gives more accurate information regarding patients’ disorders, thus facilitating proactive disease monitoring and improving disease managing outcomes.
The different interventions that patients receive according to the specific genetic diagnosis help them better manage their diseases. Personalized management strategies maximize their effectiveness. Moreover, a significant portion of the participants are identified to have potentially progressive disorders, informing their long-term care..
This finding helps in understanding disease trajectories, continuing longitudinal symptom evaluation, and reevaluating diagnoses to adjust to better and more precise treatments targeting the exact disorder. Some of the family and relatives of these participants who have no defective motor phenotypes are advised for genetic testing due to the potential risk of the mutation being familial. The earlier the diagnosis, the easier it is to treat and manage the disease.
This seems like a promising tool to help patients in getting better care, especially patients with rare diseases. It is frustrating not knowing what’s causing the symptoms and bearing the burden of dealing with the mysterious illness alone during the years without a definitive diagnosis. Genetic testing might not give the answer right away but it is revealing the root and giving comfort little by little.
- Posey, J. E. & Lupski, J. R. Genomics in Clinical Practice. N Engl J Med 388, 1619–1620 (2023).
- Butler, M. G., Moreno-De-Luca, D. & Persico, A. M. Actionable Genomics in Clinical Practice: Paradigmatic Case Reports of Clinical and Therapeutic Strategies Based upon Genetic Testing. Genes (Basel) 13, 323 (2022).
- Kim, M.-J., Yum, M.-S., Seo, G. H., Ko, T.-S. & Lee, B. H. Phenotypic and Genetic Complexity in Pediatric Movement Disorders. Front Genet 13, 829558 (2022).
- Rosello, M. et al. Hidden etiology of cerebral palsy: genetic and clinical heterogeneity and efficient diagnosis by next-generation sequencing. Pediatr Res 90, 284–288 (2021).
- Hama, Y. et al. A Novel de novo KIF1A Mutation in a Patient with Ataxia, Intellectual Disability and Mild Foot Deformity. Cerebellum 22, 1308–1311 (2023).
- Liao, P. et al. Association of variants in the KIF1A gene with amyotrophic lateral sclerosis. Transl Neurodegener 11, 46 (2022).
- Srivastava, S. et al. Molecular Diagnostic Yield of Exome Sequencing and Chromosomal Microarray in Cerebral Palsy: A Systematic Review and Meta-analysis. JAMA Neurol 79, 1287–1295 (2022).
- Gonzalez-Mantilla, P. J. et al. Diagnostic Yield of Exome Sequencing in Cerebral Palsy and Implications for Genetic Testing Guidelines: A Systematic Review and Meta-analysis. JAMA Pediatr 177, 472–478 (2023).

