#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dominique Lessard and Dr. Dylan Verden of KIF1A.ORG summarize newly published KIF1A-related research. From February 5 – April 16, 2022, a team of talented students from Columbia University’s M.A. in Biotechnology program is taking over the Rare Roundup section of the #ScienceSaturday blog! What topics do you want to learn more about? Send suggestions to our team at impact@kif1a.org.
KIF1A in the News
Dr. Arne Gennerich and Dr. Hernando Sosa awarded $100,000 XSeed Award for KIF1A research!
We offer our heartfelt congratulations to Dr. Gennerich and Dr. Sosa, faculty at the Albert Einstein College of Medicine! This year’s XSeed Award was granted to projects that apply basic science to better understand and treat neurodegenerative disorders. Dr. Gennerich is an active member of our Research Network who has published several papers on the function of KIF1A and other kinesins, and KIF1A Research Network member Dr. Sosa brings a wealth of expertise in kinesin structural analysis. Their project will use virtual, cellular, and animal models to characterize KIF1A and discover small molecules that can reverse KIF1A dysfunction. These studies are crucial for better understanding, and finding treatments, for KAND—we thank Dr. Gennerich and Dr. Sosa for their efforts and we look forward to seeing what they discover!
KIF1A-Related Research
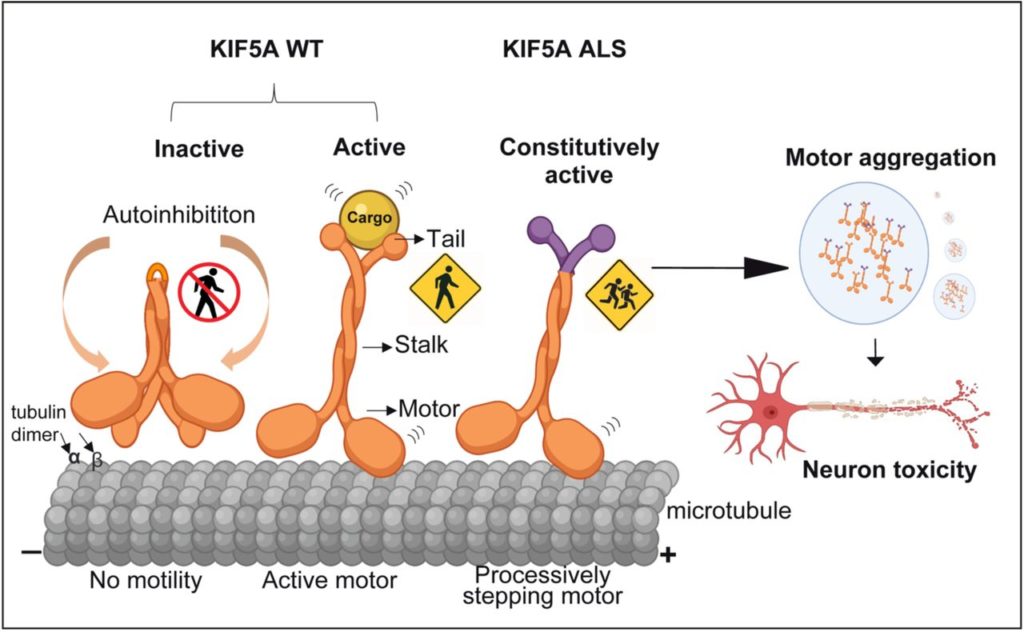
ALS-linked KIF5A ΔExon27 mutant causes neuronal toxicity through gain of function
In line with the XSeed Award announcement, today we will be sharing a recent pre-print co-authored by Dr. Gennerich! This study investigates KIF5A, a kinesin motor protein whose dysfunction is associated with amyotrophic lateral sclerosis (ALS). While it is natural to think about disorders caused by proteins that lose function, this paper describes a KIF5A mutation with increased motor activity, causing it to accumulate at the tips of neuronal processes. This overactive transport can deplete cell bodies of KIF5A, resulting in long-term transport deficits. Because kinesin motors act in pairs, a single copy of the mutant KIF5A gene can also disrupt the function of normal KIF5A proteins. This mutation also makes the protein “stickier”—clusters of KIF5A accumulate within the cell. These traits were shown to cause cell death in cultured neurons, and motor deficits or paralysis in flies.
How does this KIF5A mutation cause these problems? Under normal circumstances, KIF5A must bind to a cargo in order to activate and walk down the cell. Without cargo, KIF5A folds upon itself in a way that keeps its motor off, a process called auto-inhibition that conserves energy and retains reserves of KIF5A in the cell body for when it is needed. The ΔExon27 mutation described in this paper prevents auto-inhibition, so the motor is always on regardless of cargo. Even though KIF5A is functional, it doesn’t perform these functions in the context the cell needs. This speaks to the importance of characterizing individual KIF1A mutations in KAND research, so that our treatments are informed by the specific cellular dysfunctions our community members face. We are grateful that the expertise highlighted in this paper will be directed at KIF1A research through Dr. Gennerich and his collaborators.
Rare Roundup
Welcome to the #ScienceSaturday Takeover portion of today’s post! Meet our guest bloggers from Columbia University, Aaron, Pragya, Keyue, Rakshitha, and Hazel, here.
What is Kinesin? Ron Vale Explains
Cells are truly the eighth wonder of the world! These micron-sized wonders are capable of numerous functions, including the ability to transport goods within themselves. Cells have specialized machinery for this function and other important ones such as communication, powering, and organizing themselves. Dr. Ron Vale is a cell biologist who discovered kinesins in the 1980s. Motor proteins like KIF1A and KIF5A are members of a “superfamily” of kinesins. What do motor proteins do? In this video, Dr. Vale speaks of cellular transportation in particular and compares the cells to roads and kinesin, a motor protein, to the trucks that carry the cargo along these roads from one location to another. Dr. Vale started his journey with kinesin as a graduate student and still does not have all the answers to its mechanisms. We do know that kinesin has two leg-like structures that pull after one another and move along the cell, carrying the goods to be transported, such as protein, on a microtubule structure.
He explains that his discovery of kinesin resulted from wondering how a cell such as a neuron is one meter long and manages to transport signals/proteins from its nucleus placed along the spine to the distal tips of the cell. While this discovery gives us some insight, the scientific community has a long way to go in understanding kinesin and other motor proteins, why they behave the way they do, and how they are empowered with the cell “map” and navigate according to its needs. Studies led by KIF1A Research Network members like Dr. Gennerich and Dr. Sosa highlighted above are critically important to helping the scientific community improve our understanding of how kinesins should function so we can develop treatments to “fix” these motor proteins when things go wrong.
Fun Fact: Dr. Vale studied the axons of squids to stumble upon this discovery and believes that he was highly serendipitous in doing so!
The Drug Development Process
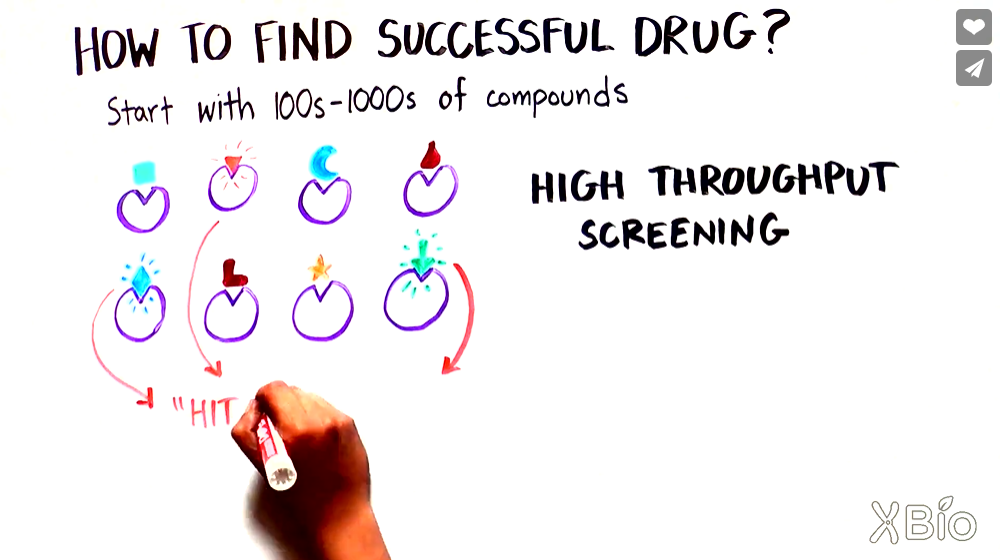
It will take a lot of hard work, a few years, and millions—or more—of dollars to develop a new drug. The video below explains the process of drug discovery by taking small organic molecules such as aspirin and caffeine as examples.
First, researchers need to spend a long time studying a disease to find a specific target suitable for the combination of compounds and cells. Then scientists will look for compounds extracted from nature or synthesized artificially that can bind to specific targets and regulate the activity of target cells; this process is called high throughput screening. But our understanding of the disease is still limited: Even after decades, we still struggle to find effective drugs to treat Alzheimer’s or Huntington’s disease, although in KAND we have the advantage of knowing the primary cause of disease—mutant KIF1A. The next step is the use of mice and other animals to test the effectiveness and safety of candidate drugs. Only after passing those tests can we apply to regulatory agencies like the U.S. FDA for the human clinical trial of an investigational new drug (IND).
The clinical trial is divided into three stages.
- A phase I clinical trial is used to test the safety of candidate drugs in humans.
- A phase II clinical trial is used to further explore the safety and effectiveness of the drug.
- A phase III clinical trial needs to recruit more patients to evaluate the effectiveness of trial data before the drug can move forward.
If the trial data meets the requirements, we can apply for approval with regulatory agencies. However, sometimes for exceptional cases such as epidemics or severe life-threatening diseases, the FDA allows us to promote the clinical trial and marketing process of drugs quickly. The effectiveness and safety of drugs are still regulated after listing to avoid rare side effects endangering patients’ lives. These steps are necessary to ensure that our therapeutics help patients without doing harm, but the length of this process can be frustrating—this underlines KIF1A.ORG’s urgency in promoting as many steps of the therapeutic pipeline as possible, as soon as possible.
Fun Fact: the organization that produced this video, XBio, was founded by Dr. Ron Vale, who discovered kinesins!
A call for an integrated approach to improve efficiency, equity and sustainability in rare disease research in the United States
Recent technological breakthroughs in genome sequencing and gene therapy brought hope for rare disease patients, which account for 10% of the US population. However, current research largely relies on single-disease approaches to study individual rare diseases. This limits our understanding of the connections across rare diseases and thus the ability of scientific advances to benefit a larger group of patients. Why is it important to think about rare diseases as a whole rather than simply focusing on one of them to find an effective treatment or cure? Coordination across research in rare diseases with similar causes or symptoms increase efficiency of outcome measure development and accelerate the pace of drug approvals. In addition, it could also help us build a broader diagnostic and health service system that covers more than one type of rare disease. In this way, we can present more convincing epidemiological evidence on the collectively tremendous burden of rare diseases to decision makers and urge them to reconsider current health policy and funding decisions.
Single-disease approaches are also prone to deepen inequalities in terms of allocation of research resources. For example, research in cystic fibrosis primarily affecting Euro-Americans is achieving more therapeutic progress than sickle cell disease primarily affecting African-Americans, despite the higher prevalence of the latter. Even within cystic fibrosis, very few therapies are effective for genetic variants primarily affecting patients with Hispanic ancestry, despite the higher death rate in this group. Genomic information of non-Euro-Americans is also under-represented in databases due to less accessibility to scientific research, hindering their genetic diagnostics. Taken together, a more integrated approach could raise awareness of the immense cumulative burden of rare diseases, address the inherent inequities through data and resource sharing, and facilitate clinical translation of remarkable advances in biotechnology.
EURORDIS-Rare Diseases Europe – Launches Ukraine Resource Center
Amidst the war in Ukraine, EURORDIS-Rare Diseases Europe has released a statement and outlined the ways in which they will continue to support rare disease patients in Ukraine. Lack of mobility and dependence on regular care make rare disease patients especially vulnerable due to the destruction of infrastructure. While it is clear that significant challenges lie ahead, EURORDIS commended a host of individual rare disease organizations who have continued to support and assist the rare disease community, and mobilized policy advocacy and legislation at the national level. Lastly, in their statement, EURORDIS calls upon the EU, the UN, WHO Europe, other UN agencies, humanitarian organizations, and the international community at large, to protect those with rare diseases during this crisis.
Along with their statement, EURORDIS launched a webpage specific to the situation. The webpage, available in both Ukrainian and English, contains links and documents that may be useful, and a section of examples, stories, and testimonies about the situation for rare disease patients and their families. On top of this, there are country/region-specific sections for Ukraine, nearby countries, and the EU bloc with relevant information and resources. The Ukraine section outlines EURODIS’ three immediate priorities:
- To link with aid agencies and relevant patient organizations in order to identify how vulnerable people with rare diseases can leave (quickly and safely) and how to get essential supplies in,
- Ask Ukrainian authorities to recognize the specialized needs of vulnerable rare disease patients including an exemption that would allow men with a son, daughter, or partner with a rare disease to leave the country,
- Establish a surveillance and monitoring mechanism to track the needs and contacts of the rare disease community to ensure the most efficient public health response.
The war in Ukraine has the potential to escalate to a humanitarian crisis. EURORDIS’ action, and collaboration with other medical and rare disease organizations looks to ensure that those with rare disease are not left behind.

