Translate this page:
We did it! In less than a month, we raised $100,000+ USD to launch the Treatment Accelerator Program (TAP) to expedite the search for treatments for our superheroes. So, what does all of this mean? Let’s answer some of your questions about what’s next to come!
What is TAP?
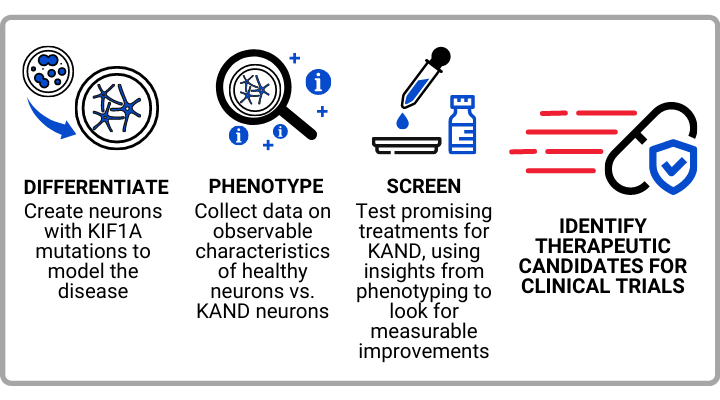
The Treatment Accelerator Program will allow us, through a Contract Research Organization (CRO), to create experimental models of KAND to test potential therapeutics. As scientists identify drugs that they think have potential to treat KAND, we can’t just give the drug to our kids first, unless it’s a common drug with a known safety profile. Before we get to human clinical trials, we need to “model” KAND in the lab. This can be done through animal models, like mice with KAND. But it can and should also be done in cellular models! Using blood samples donated by patients, scientists can turn them into something called induced pluripotent stem cells (iPSCs). iPSCs can then be turned into specific cell types. In our case, scientists will turn the iPSCs into neurons with KAND. Once the cells are turned into neurons, scientists can rigorously examine the cells to see how KAND affects their function. They will look for things we can measure so that when we introduce a drug to the cell, we can see if it’s having a positive or negative effect, or no effect at all. There are thousands of possibilities as far as what to measure, and this project will help identify the most effective measurements. Once we have a strong system in place, we can introduce potential drugs.
Who is NeuCyte?
KIF1A.ORG is sponsoring NeuCyte to be our first TAP partner. NeuCyte a biotechnology company solely focused on neurological diseases and is known for their cell-based target identification and drug discovery platforms. Learn more about our partnership here. While we’ve been preparing many months for this partnership and scoping out the project’s aims, the project launched in September 2021. Click here to read about an update on the initial stage of the project from NeuCyte, which was presented during KIF1A.ORG’s November 2021 Research Roundtable meeting.
How is TAP used to find treatments?
We have identified 3-5 drugs that scientists think might be able to help treat KAND. These drugs would not be a cure— we need gene editing for that. But our aim is to find a drug that can help stop or reduce the harmful effects of KAND to buy us time and some relief before gene editing is ready. The number of potential drugs we want to test will continue to grow, so we’ll need to continue funding this work as we want to run more and more drugs through the system. No matter how well we might think a drug would work in theory, we won’t really know until we get it in the lab. It’s an ongoing, trial and error process. But this is exactly how pharmaceutical companies discover treatments for diseases. We’re taking control of the process by hiring a lab to create this system. What’s great about that, is this will be an open-source tool for any academic institution, or pharmaceutical company or biotech to test their drugs on KAND. This will attract more scientists and companies to work on KAND treatment.
What drugs are being tested?
Right now we have 3-5 drugs that we want to run through the system. These are drugs that have been developed to treat other similar neurodegenerative disorders. They will not target KIF1A directly, but have potential to help treat the harmful effects of KAND. Because they don’t target KIF1A directly, the drug would be more likely to benefit most or all of our patient population, despite the wide range of variants associated with KAND. Before we can determine when the first clinical trial would be, there are still investigations to be completed before we are confident that any treatment we’re exploring should move forward to human trials. Our main goal in 2021 has been to identify at least one potential treatment with a solid clinical proof of concept by the end of the year, but there are no guarantees. The more “shots on goal” we make, the better position we are in to discover something with solid potential.
Is it guaranteed to work?
In the world of science, nothing is guaranteed. On the flip side, in the world of science, anything is possible. Because these drugs to be tested first were developed to treat other similar neurodegenerative disorders like KAND, we are hopeful it will yield results beneficial for KAND patients. You can learn more about our therapeutic strategy here.
Want to learn more?
Check out Dr. Dominique Lessard’s Treatment Accelerator Program Update from our 2021 KAND Conference to hear an overview of TAP! You can also read about our Treatment Accelerator Program partner and related blog post here!


