Welcome to the KIF1A Glossary. This resource is intended to help the community understand scientific and medical terminology that you will encounter in literature, and communication with researchers and physicians.
To find a specific term or word, simply search this page and click on the dropdown to expand the appropriate section. Not finding a term you’d like to learn more about, or need help understanding something? Send an email to impact@kif1a.org and our team will get right back to you. We might be rare, but you are not alone.
Basic Science Terms
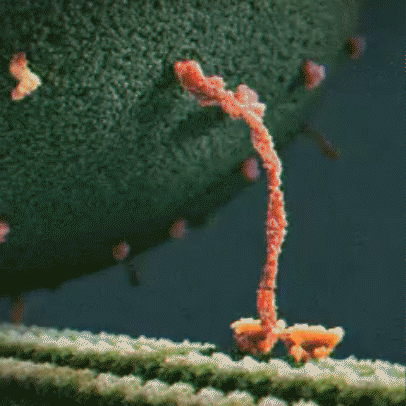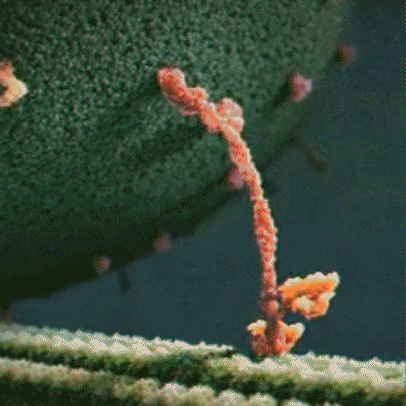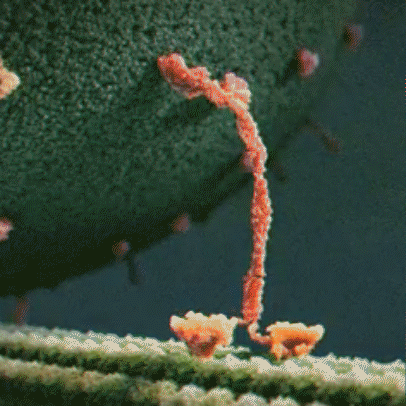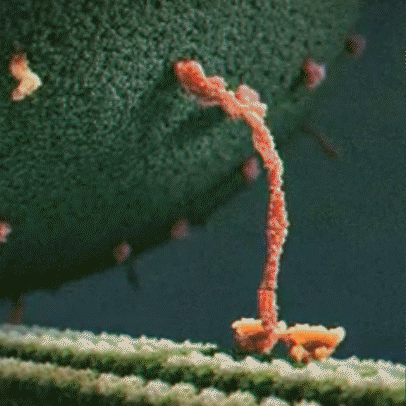
Molecular motor: Molecular motors, or motor proteins, use energy released from ATP hydrolysis to transport cargo along cytoskeleton filaments. There are several different types of molecular motors, including kinesin.
Kinesin: Kinesins are a type of motor protein that transport cargo within the cell. They transport cargo such as membrane-bound vesicles, proteins, and organelles. There are about 40 different types of kinesins, including KIF1A, which is found predominantly in the brain. Driven by energy provided by the hydrolysis of ATP, they move along microtubules in a walking motion. The other end of the protein has a binding domain, which binds to the cargo the kinesin is transporting.
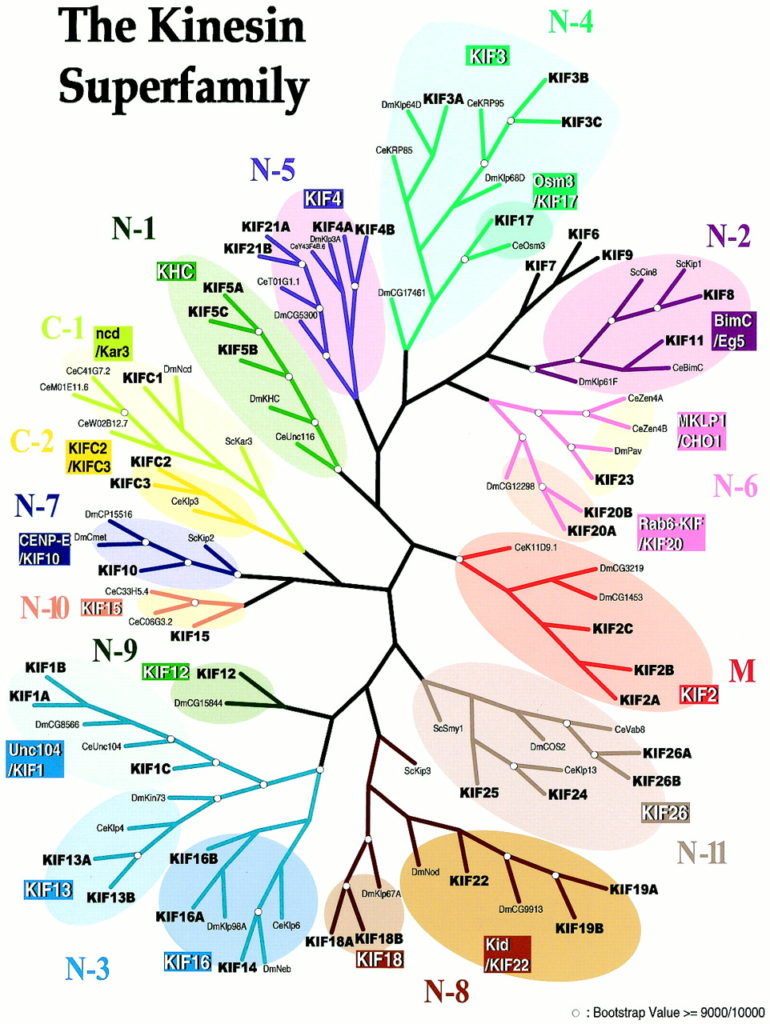
KIF: KIF refers to proteins in the kinesin superfamily. KIFs are a family of motor proteins that are essential for cellular functions. Using microtubules as roads, they transport cargo such as organelles and macromolecules within the cell and along neuronal axons and dendrites. They have important roles in neuronal development and brain wiring. Many KIFs are found in the brain, which requires a precise, regulated, and complex transport system. KIFs also help in chromosomal and spindle movement during mitosis (a type of cell division) and meiosis (cell division in reproductive cells). For a more technical description of KIFs, please see this link.
Cellular cargo: Kinesins transport cellular cargo within cells and neurons. There are several different types of cargo, including membrane-bound vesicles, proteins, and organelles.
Molecular motor | Kinesin | KIF | Cellular cargo
hydrolysis to move along a microtubule
KIF1A motor domain: The motor domain connects to the stalk. Each motor domain has a binding site for the microtubule and another for ATP. The KIF1A motor domain contains an important stretch of particular amino acids that bind to the microtubule that KIF1A travels along. This part of the motor domain is called loop 12, also known as the K-loop. The K-loop forms the “binding surface” and interacts with tubulin (β-tubulin and α-tubulin make up the microtubule) through the glutamate-rich C-terminal tail of β-tubulin (microtubule). The K-loop of KIF1A motor domains enable the motors to work in teams. The unique properties of the KIF1A K-loop cause the KIF1A motor domain to bind the microtubule approximately 200 times stronger than other kinesin motors. The energy from ATP hydrolysis causes the globular heads (the motor domains) to move in relation to the neck that connects the stalk and the motor domain, resulting in movement. Mutations in the motor domain can cause issues with energy use or retention, or it can cause the motor domain to have issues binding to the microtubule.
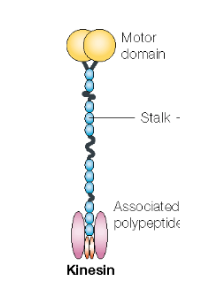
KIF1A stalk region: The KIF1A stalk is a long stretch of protein between the motor domain and the cargo-binding domain. The stalk is important for the dimerization of KIF1A, or bringing two copies of KIF1A together so that two motor domains can regulate movement along microtubules. It has a number of special features and important functions, and it helps provide structure for the KIF1A protein. For example, it contains the coiled-coil domain, the forkhead-associated domain, and the neck coil.
Coiled-coil domain: The coiled-coil domain is part of the stalk. It is important for the dimerization of the KIF1A protein (dimerization brings two copies of KIF1A together so that two motor domains can regulate movement along microtubules) and the motor domain. The coiled-coil region is also responsible for autoinhibition of KIF1A, meaning that KIF1A is in a “turned off” state until it is activated. It blocks motor activity until a cargo binds and releases the inhibition.
KIF1A cargo-binding region: The cargo-binding domain is the region of KIF1A that binds to the cargo that needs to be transported. A mutation in this region can make it difficult to attach to the cargo, or it could cause the cargo-binding region to hold on too tightly. In either case, mutations in this region can interfere with the transportation of the cargo.
KIF1A neck coil: The neck coil facilitates dimerization in KIF1A. It is the beginning of the stalk, and it connects to the motor domain.
Forkhead-associated domain: Another part of the stalk is the forkhead-associated domain (FHA domain), which is a region of KIF1A that interacts with other proteins in the neuron. The FHA domain recognizes phospho-peptides in several important proteins that provide structural support to the KIF1A protein, and are involved in regulating cargo interactions.
KIF1A motor domain | KIF1A stalk region | Coiled-coil domain | KIF1A cargo-binding region | KIF1A neck coil | Forkhead-associated domain
Microtubule: Microscopic hollow tubes made up of a protein called tubulin, microtubules are one of three main components of the cytoskeleton. Their length can change with the addition and removal of tubulin. Microtubules extend throughout the cell, keep its organelles in place, and help the cell maintain its shape. They are also important in the transportation of materials in cells, and they act as roads for motor proteins such as kinesin. Microtubules also assist in cell division.
Cytoskeleton: The cytoskeleton is made up of microtubules, actin filaments (also known as microfilaments), and intermediate filaments. Cells are highly structured, and they would not be able to function properly without the cytoskeleton which maintains the cell’s shape and organization. It also helps the cell carry out cellular functions, such as cell division and movement.
Microtubule | Cytoskeleton
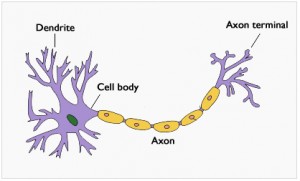
Neuron: Neurons, or nerve cells, are considered the building blocks of the nervous system. They send and receive messages from the brain, and communicate information throughout the body. Electrical information is sent in the form of action potentials (electric impulses). Although neurons can vary in size and structure, most neurons are composed of a cell body, dendrites, and an axon. The cell body (also known as a soma) contains a nucleus and specialized organelles that are located in the cytoplasm and perform specific cellular functions. Neurons receive and process signals at the dendrites, which branch out from the cell body. These signals are also processed in the cell body and then transmitted along the axon, a long structure that carries messages to the axon terminals where it is transmitted to another neuron. The area where information is passed from one neuron to another is called a synapse.
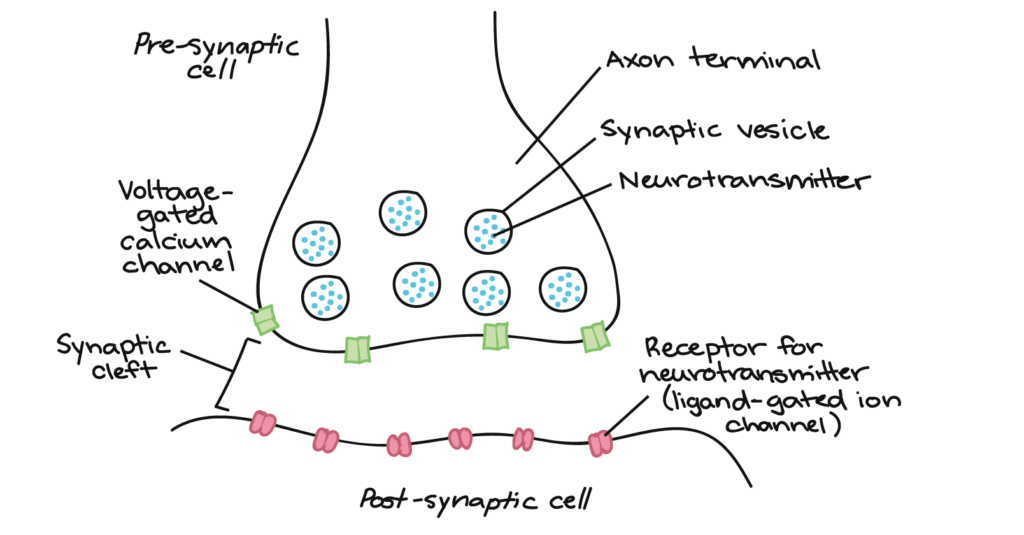
Synapse: Information is transmitted from one neuron to another through the synapse, or neuronal junction. The neuron that sends the signal is called the presynaptic neuron, while the one that receives the signal is called the postsynaptic neuron. The pre and postsynaptic neurons are separated by a microscopic space where chemical signals (neurotransmitters) can pass and bind to receptors on the postsynaptic cell.
Synaptogenesis: Synaptogenesis is the development of synapses between neurons.
Axonal transport: The process where proteins, synaptic vesicles, and materials produced in the cell body are transported along the axon by kinesin motors and dynein.
Neuron | Synapse | Synaptogenesis | Axonal transport
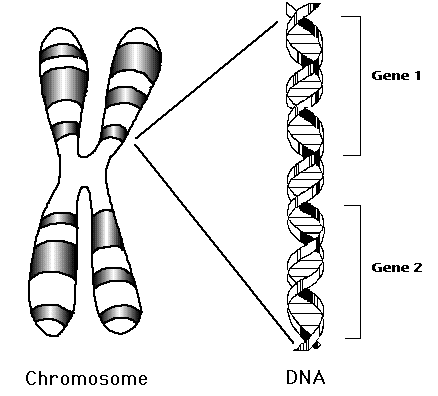
Gene: Humans have approximately 20,000 genes. Genes are a unit of hereditary material. Simply put, genes are instructions that determine the specific traits of an organism, such as its appearance and behavior. Genes are found in chromosomes, which are made of tightly bound DNA (Deoxyribose Nucleic Acid) and protein. DNA provides instructions for living things to develop and function. The information found in DNA is stored as code in four chemical bases: adenine, thymine, guanine, and cytosine. Different sections of chromosomes are called genes. Proteins are encoded by genes, which make up and perform most of life’s functions, and determine different traits, such as height or eye color in individuals. They also pass hereditary information from parent to offspring. Offspring may inherit the likelihood of getting certain diseases from parents.
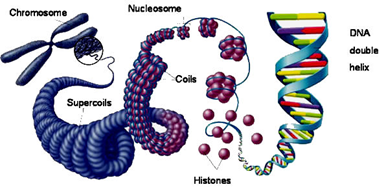
Chromosome: Located in the nucleus, chromosomes are made of tightly bound DNA wrapped around proteins called histones. Histones help give structure to chromosomes. Specific sections of chromosomes contain genes. KIF1A is located on chromosome 2. Humans have 23 pairs of chromosomes for a total of 46. Offspring receive half of their chromosomes from their mother, and the other half from their father.
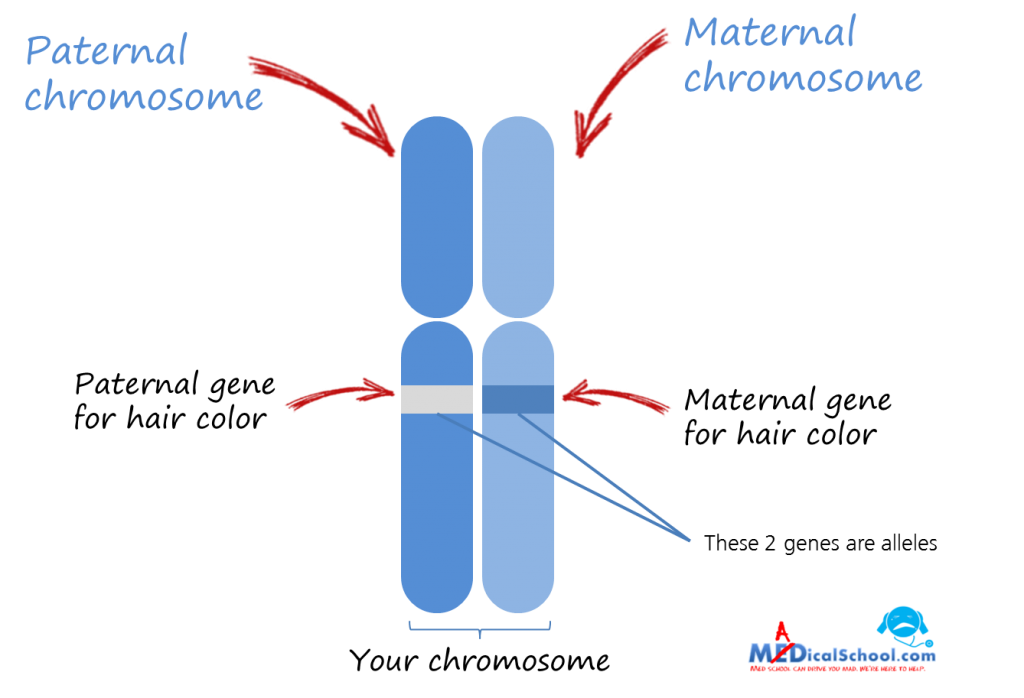
Allele: Alleles are variants of a gene that are located in specific locations on chromosomes. For example, alleles for hair color code for different colors such as blonde, brown, or red. When someone has two different types of alleles (such as an allele for blonde hair and one for brown) they are heterozygous. While if they have two of the same alleles, it is considered homozygous. One allele is inherited from each parent. They are either dominant or recessive, and are often represented with lowercase or capital letters (lower case represents a recessive allele while capital letters represent a dominant allele). The recessive trait is only expressed when the individual’s genotype is homozygous, meaning they have two recessive alleles, one from each parent. Dominant alleles only need one copy to be expressed.
Genetics: Genetics is the study of heredity and DNA. It is the study of genes and how they are inherited from parents to offspring.
Genome: Every human has one genome, 46 chromosomes, and 20,000 genes. A genome contains all of the information an organism needs to grow and develop. It is an organism’s complete genetic code.
Gene | Chromosome | Allele | Genetics | Genome
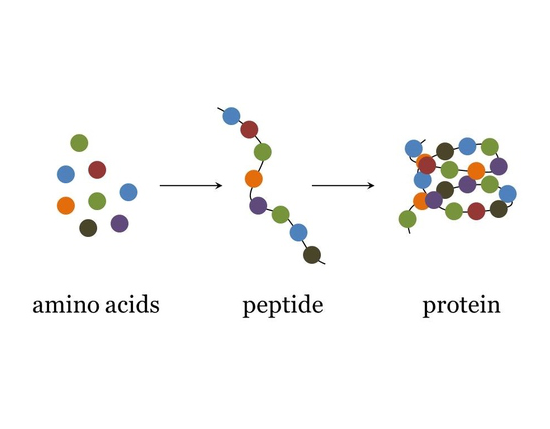
Amino acid: Amino acids are monomers (individual building blocks) that make up proteins. There are 20 types of amino acids. Amino acids bind together to form proteins during protein synthesis. Different proteins have different sequences of amino acids, which results in different protein structures and functions. Just like how many different sentences can be formed using the limited letters in an alphabet, many different proteins can be created with amino acids.
Mutation: Mutations are changes in the DNA sequence that alter gene expression and may cause genetic disorders. They change DNA’s code, which can result in dysfunctional or absent proteins. However, mutations do not always result in genetic disorders, and they are actually very common. Everyone has mutations, which create genetic variation and result in evolution. Mutations can be inherited, can occur spontaneously, or they can form due to environmental factors such as radiation, chemicals, or smoking. In the case of KAND, mutations can be de novo or inherited. The word mutation is often used interchangeably with variant.
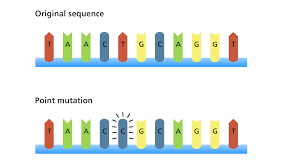
Point mutation: A point mutation occurs at a specific point in the genetic code where there is a change to one or more nucleotides. Substitution is a type of point mutation where a nucleotide is replaced with another nucleotide. For example, if adenine were replaced with guanine. There are several possible consequences to a point mutation.
If the mutation does not result in a different amino acid (the building blocks of protein), it is called a silent mutation.
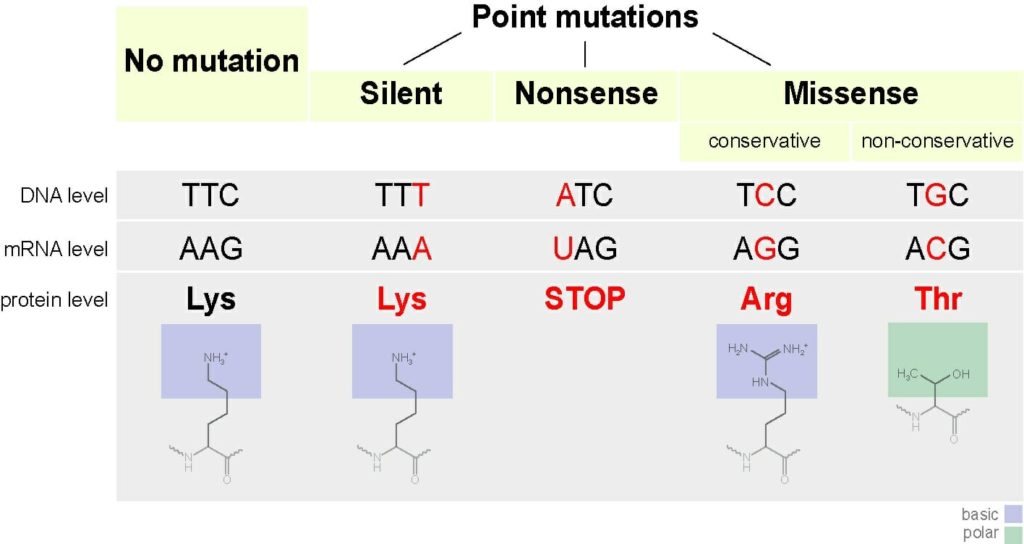
A missense mutation occurs when a point mutation results in an altered amino acid in the protein structure. The missense mutation is considered non-conservative if the new amino acid has different properties than the one being replaced that alter the structure and function of the protein. It is conservative when the new amino acid has similar properties to the one being replaced. This may result in a loss of function (a compromised or disfunctional protein); however, the change may be slight.
If the mutation codes for a stop codon, it is called a nonsense mutation. Stop codons code for the end of the protein production. Nonsense mutations result in the premature termination of protein production and usually a loss of protein function.
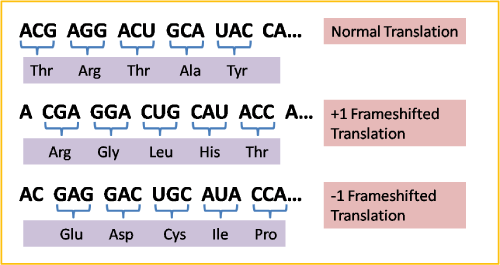
Frameshift mutations are also considered point mutations. They occur when a nucleotide is added or deleted, which changes the order of the nucleotides, and thus alters the protein.
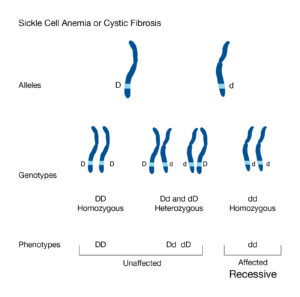
mutation in order for it to be expressed.
Recessive mutation: In order for a recessive mutation to be expressed, both alleles must carry the mutation. This means that both parents must carry the mutation. Recessive mutations lead to loss of function. Loss of function mutations result in dysfunctional or compromised proteins.
Dominant-negative mutation: A dominant-negative mutation (also known as an antimorphic mutation) causes a change to the wild-type gene (the natural gene). The mutated gene interferes with the function of the normal gene.
Gain of function: Gain of function mutations are less common than loss of function mutations. Gain of function mutations result in a new function for a mutant protein.
Loss of function: Loss of function mutations result in a reduction or complete loss in a protein’s ability to function. Loss of function mutations are generally recessive mutations. KAND compromises the ability of the KIF1A protein to function properly.
De novo mutation: De novo mutations happen spontaneously in the affected individual. They are not inherited, and are new mutations that either form in the germ cell (reproductive cells) of the parent or in a fertilized egg during early development. KAND can result from a de novo mutation.
Amino acid | Mutation | Point Mutation |Recessive mutation | Dominant-negative mutation | Gain of Function | Loss of function | De novo mutation
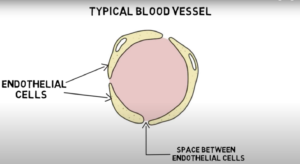
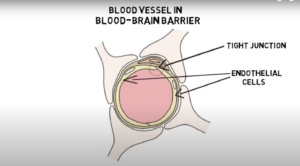
Blood-brain barrier: The blood-brain barrier is a semi-permeable, protective barrier between circulating blood and extracellular space (the space between cells that contains substances such as protein and hormones) of the brain. It defends against harmful pathogens and toxins in our blood while allowing nutrients to the brain. The barrier’s main component is formed by the endothelial tight junction. Endothelial cells line the walls of blood vessels. In other blood vessels in the body, there are small gaps between endothelial cells. However, in the blood-brain barrier, the endothelial cells are packed closely together, forming a tight junction that only allows small molecules, fat-soluble molecules, and certain gasses to pass freely without protein assistance. Although it provides essential protection of the brain, the blood-brain barrier makes it difficult for treatments and medicine to reach the brain.
Neuroplasticity: Neuroplasticity is the brain’s ability to change and develop new neural pathways in response to new information and experiences. It is the brain’s ability to adapt. For example, when learning new information, new connections between neurons are formed.
Neurodegeneration: Neurodegeneration is the progressive deterioration of nerve cells that is present in neurodegenerative diseases.
Neurodegenerative: Neurodegenerative diseases cause cells in the central nervous system to die or become dysfunctional. Usually, neurodegenerative disorders worsen over time.
Blood brain barrier | Neuroplasticity | Neurodegeneration | Neurodegenerative
Clinical Terms
Coding DNA: Coding DNA is transcribed and translated. Also known as an exon, it is DNA that codes for proteins. Coding DNA is separated by introns, which are not expressed as proteins.
Genetic testing: Genetic tests are used to identify mutations in DNA, which can identify or eliminate possible genetic disorders. There are several types of genetic testing, including whole exome sequencing, whole genome sequencing, and multiple gene sequencing panel tests.
Multiple gene sequencing panel tests (panel tests) look for mutations in several genes. Panel tests sequence the exons in specific genes based on the medical concern. This means that the nucleotides in the coding regions in genes (exons) are ordered. Any mutations in the sequenced exons would be identified.
Whole exome sequencing involves sequencing the exons of all genes. Exons are genes that are expressed. Non-coding regions of genes are called introns. Collectively, exons make up the exome. They account for about 1-2% of DNA; however, approximately 85% of genetic mutations are found in the exome. Sequencing exons means that the order of the nucleotides in all of the exons are determined, which reveals any possible mutations in the exome. Sequencing the exome requires a sample of DNA which usually comes from blood samples. It typically takes weeks or months to get the results.
Whole genome sequencing is another option for genetic testing. It involves sequencing and ordering all 3 billion nucleotides in DNA. This is a more thorough test than whole exome sequencing, and sequences the whole genetic code rather than just the exome. This test is usually used if whole exome sequencing does not yield any results.
Electroencephalogram (EEG): An EEG test can diagnose disorders that affect the brain. It measures the electrical impulses in the brain, and can identify abnormal brain activity. Neurons communicate through electrical impulses. An EEG can detect abnormalities in these electrical pulses. During the test, the patient has electrodes with wires attached to their scalp in order to detect the brain activity.
Coding DNA | Genetic testing | Electroencephalogram (EEG)
Variant: A variant is a change in an individual’s genetic code. A variant can alter gene expression and result in a genetic disorder. They change the DNA code, which can result in dysfunctional or absent protein. Mutations can be inherited, occur spontaneously, or they can form due to environmental factors such as radiation, chemicals, or smoking. In the case of KAND, mutations can be de novo or inherited. The word variant is often used interchangeably with mutation.
Pathological variant: A pathological, or pathogenic, variant a is mutation that makes individuals more susceptible to certain disorders. These variants make an individual more likely, but not certain, to develop a disorder.
Zygosity: Zygosity is the similarity of certain genes within individuals or between twins. For example, diploid organisms (organisms with two sets of chromosomes) are heterozygous when there are two different versions (or alleles) of a gene. When the alleles are the same, it is called homozygous. If there is one allele missing, the organism is hemizygous, and if both alleles are missing, it is nullizygous. In relation to twins, twins that are monozygotic began as a single egg, which later split into two embryos. They are dizygotic if two independent eggs are fertilized.
Variant | Pathological variant | Zygosity
Pre-clinical research: Scientists and clinicians use a process to find and develop potential medicines. They start by understanding patients’ needs, and the biological and genetic details of the disorder they are trying to treat. Next they test hypotheses and potential medicines in “preclinical models.” They identify potential medicines and test them for effectiveness and safety in laboratory settings before the medicine can be tested in people. There are two types of preclinical research: in vitro and in vivo. In vitro experiments are performed in a dish or outside of an animal by testing proteins or cell culture. In vivo experiments involve administering the treatment to animals such as mice.
Clinical trial, phase 1: Clinical research is the testing of a potential treatment in people. Traditionally there are three separate “Phases” or types of clinical trials that scientists and clinicians use to prove a treatment is safe and effective in people. In the first kind of trial, Phase I, a treatment is given to people for the first time. Investigators test to confirm whether a potential treatment is safe and well tolerated. Safety is the main concern during this phase. They also determine the highest dose that can be safely administered to a person. Even though the treatment has been tested in animals, human side effects cannot always be predicted. The first patients begin with low doses, which are slowly increased as long as the side effects remain minimal. The effect of the treatment on the body is closely observed throughout the process.
Clinical trial, phase 2: The purpose of Phase II clinical trials is to explore the effectiveness of a medicine and to find the appropriate dose for the medicine. Phase II trials typically involve more people. Next, investigators move to Phase III clinical trials, where larger numbers of patients are involved and the objective is to confirm efficacy and safety of the medicine.
Clinical trial, phase 3: Phase 3 compares the treatment’s safety and efficacy to other treatments for the same condition while continuing to monitor the drug’s safety and benefits. Investigators need to demonstrate that the treatment is at least as safe and effective as existing treatment options. The number of patients from phase two are increased.
Baseline: A baseline is an individual’s initial measurement taken early on in a condition. The baseline is used for comparison over time to observe the progression of the condition.
Pre-clinical Research | Clinical trial, phase 1 | Clinical trial, phase 2 | Clinical trial, phase 3 | Baseline
Hypotonia: Hypotonia is a condition that causes decreased muscle tone. It can be a condition by itself (benign congenital hypotonia) or it can be caused by another disorder that affects the brain, central nervous system, or muscles. Symptoms in infants and children may include poor head control, delayed motor skills, such as crawling, and a delay in fine motor skill development.
Hypertonia: Hypertonia is a condition where someone has too much muscle tone in their body. This can be caused by issues in the central nervous system, which results in a lack of control over muscle tone. This makes it difficult to move around properly and can result in stiff movements and issues with balance, walking, and reaching. There are two types of hypertonia: spastic hypertonia and dystonic hypertonia. Spastic hypertonia causes random and uncontrollable muscle spasms. This is primarily caused by spine or brain injuries. Dystonic hypertonia results in a lack of flexibility. This results in an inability to stretch muscles.
Ataxia: Ataxia is a degenerative disease relating to the nervous system that causes a loss of control of bodily functions. Most causes of ataxia involve neurodegeneration in the cerebellum. It can lead to issues with the fingers, hands, limbs, speech or eye movements. This may result in poor coordination, slurred speech, tremors, trouble swallowing, fine motor skill deterioration, or a lack of balance.
Muscle tone: Muscle tone is the contraction of muscles while at rest. It maintains balance and posture, generates heat, and allows for fast reactions.
Epilepsy: Epilepsy is a neurological disorder that causes recurring seizures. Seizures are caused by a burst of electrical activity in the brain. There are many different types of seizures and symptoms that depend on what part of the brain is affected. Some common symptoms include memory lapses, loss of consciousness, staring spells, difficulty speaking, dilated pupils, drooling, loss of bladder control, rigid muscles, tremors, chewing movements, and repeated movements. However, although there are common symptoms for different types of seizures, not everyone will experience the same symptoms. The two main categories are generalized seizures and focal seizures.
Generalized seizures affect the whole brain. There are six types of generalized seizures: absence seizures, tonic seizures, atonic seizures, clonic seizures, myoclonic seizures, and tonic-clonic seizures. Absence seizures cause a blank stare. Tonic seizures result in muscle stiffness. Atonic seizures cause a loss of muscle control resulting in sudden falls. Clonic seizures are identified by jerky muscle movements in the face, neck and arms. Myoclonic seizures cause sudden twitching of arms and legs. Tonic-clonic seizures cause stiffening of the body, shaking, loss of bladder of bowel control, and a loss of consciousness.
Focal seizures, also known as partial seizures, only affect part of the brain. There are two main types of focal seizures: simple partial seizures and complex partial seizures. A simple partial seizure does not cause a loss of consciousness, and symptoms can include dizziness, tingling or twitching limbs, and altered senses such as taste, smell, sight, hearing, or touch. Complex partial seizures cause a loss of consciousness, and possible symptoms include unresponsiveness, staring blankly and repetitive motions. Mild seizures can be a couple of seconds of confusion and a lack of awareness. As a result, they can be difficult to identify.
Some people have certain triggers that can cause a seizure. They may also have warnings called auras at the onset of a seizure and feelings of disorientation or illness may persist after the seizure. During a seizure, make sure that the surroundings are safe, and wait for the seizure to pass. Do not restrict their movements, put anything in their mouth, or move them until it has passed. If a seizure lasts more than 5 minutes, or if 2 or more consecutive seizures occur, it requires medical attention.
Visit https://www.epilepsy.com/ for detailed information on various seizure types, seizure safety, and more.
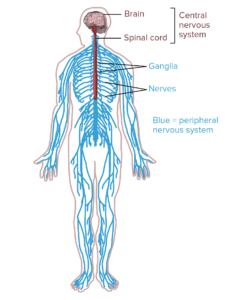
system and the central nervous system.
Neuropathy: Peripheral neuropathy is caused by damage to peripheral nerves (nerves outside of the brain and spinal cord). The peripheral nervous system sends information to and from the brain and spinal cord to the rest of the body. Peripheral neuropathy can cause pain in the body, especially the hands and feet. Symptoms vary based on the types of nerves affected by the condition. There are three main types of nerves: sensory nerves that receive sensation, motor nerves that control muscle motion, and autonomic nerves that deal with functions such as blood pressure or digestion. Symptoms include, jabbing or burning pain, gradual numbness in feet or hands, sensitivity to touch, lack of coordination, muscle weakness, heat intolerance, digestive issues, or dizziness.
Optic nerve atrophy: the optic nerve sends images from the retina (a layer of tissue in the eye) to the brain. Optic atrophy is the loss of nerve fibers. If the nerves become damaged, the brain does not receive all of the visual information from the retina. This results in blurred vision, abnormal side vision, abnormal color vision, and decreased brightness.
Cortical vision impairment: Cortical vision impairment (CVI) is caused by damage to the visual centers of the brain, causing issues with the communication between the eyes and brain. There are no physical issues with the eyes, but the brain is unable to interpret what is being seen. Some symptoms of children with cortical vision impairment include having delayed visual responses, preference for specific colors, issues with seeing far away, or visual field preference (for example, some children may see better out of their periphery).
Cerebellar atrophy: Cerebellar atrophy occurs when neurons in the cerebellum (area of the brain that controls balance and coordination) degenerate and die. Common symptoms include an unsteady, lurching gait, tremors in the torso of the body, slow, unsteady movements of the arms and legs, slurred speech, and nystagmus (rapid, uncontrolled and jerky movement of the eye that can affect sight and coordination).
Spasticity: Spasticity is a condition related to abnormal muscle tightness. The contracted muscles restrict movement, speech, and gait. It can affect any muscle in the body, but typically the leg muscles are most common. Symptoms include muscle stiffness, muscle spasms, muscle and joint deformities, and muscle fatigue. If left untreated, it can also cause urinary tract infections, chronic constipation, fever, or frozen joints. Spasticity is caused by damage to the brain, spinal cord or motor nerves.
Hypotonia | Hypertonia | Ataxia | Muscle tone | Epilepsy | Neuropathy | Optic nerve atrophy | Cortical vision impairment | Cerebellar atrophy | Spasticity
Therapeutic/Development Terms
Gene therapy: Gene therapy is an experimental treatment for diseases. It involves introducing, changing, or removing genetic material in the cell. If there is a faulty gene, scientists can replace it with a functioning one, introduce new genes, or turn off faulty genes. New genetic material can change how a protein is produced in the cell. For example, if a new gene were introduced to the cell, it could reduce the level of faulty proteins, increase the level of functioning proteins, or introduce a modified protein altogether. A vehicle called a vector carries the genetic material and inserts it into the cell. There are two types of gene therapy: in vivo and ex vivo. In vivo gene therapy is used to insert the genetic material directly into the cell in the body, while in ex vivo gene therapy, the cells are removed from the body and returned to the patient after the genes have been added to the cell.
Gene editing: Gene editing, or genome editing, is a developing approach that can be used to add, remove, or alter DNA. It involves making precise changes in DNA by using a protein, called engineered nucleases, to cut the DNA in a specific spot. This method can be used to treat disorders. An engineered nuclease is made up of a nuclease which cuts the DNA, and a DNA targeting part that directs the nucleases to a certain DNA sequence. The cell will naturally repair the cut in the DNA, changing the sequence. There are several types of genome editing. It can be used to make small changes in the DNA, remove a section of the DNA, or insert a section of DNA. There are also multiple types of engineered nuclease that mainly differ in how they know what DNA to cut. CRISPR is the most common system used for gene editing.
CRISPR: CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is used for research and is being explored as a potential medicine. Simply, CRISPR enables scientists to silence a gene, fix a gene, or replace a gene. CRISPR technology includes different modules of DNA-interacting domains such as Cas9 (CRISPR-associated protein 9) that can identify specific DNA sequences, cut DNA, replace, change, or stop gene expression.
Small molecule drug: A small molecule drug can enter cells easily because of its low molecular weight. Many drugs are small molecule drugs, including common drugs like asprin.
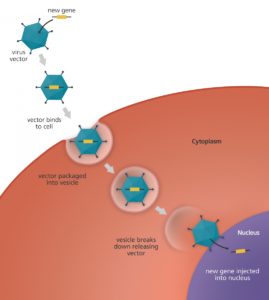
Viral vector: Viral vectors are vehicles that carry and insert genetic material into the cell. Viruses attack their host by inserting their genome into cells. By modifying viruses and replacing the disease-causing genes with the intended genetic material, viruses are used as viral vectors in gene therapy. In most cases, DNA injected directly into the cell does not function, which is why viral vectors are used to insert DNA into the nucleus.
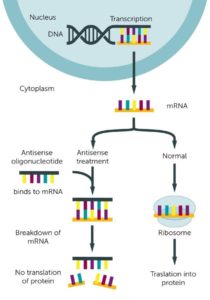
Antisense oligonucleotide: Antisense Oligonucleotides (ASOs) are small sections of DNA or RNA that bind to a specific RNA sequence to prevent protein synthesis. In the process of protein synthesis, DNA is converted to RNA, which is then translated into protein. Antisense oligonucleotides bind to RNA and prevent the production of protein. This can prevent the expression of a mutant protein.
Base editing: Base editing is a new gene editing method that can convert a base into another base. For example, it can convert the base adenine into the base guanine. This can fix a point mutation rather than disrupting an entire gene or creating unwanted byproducts or mutations. Point mutations are the causes of many genetic diseases.
Gene therapy | Gene editing | CRISPR | Small molecule drug | Viral vector | Antisense Oligonucleotide | Base editing
Assay: An assay is a test used to determine the presence, amount, or activity of an analyte (for example mutant KIF1A proteins). It can be a medicine, biochemical substance, or a cell. There are different kinds of assays. For example, it can be in a mouse (in vivo) to test drugs or medicines, or it can be cell-based, such as through cell lines (cells extracted from the body and grown in the lab). Other examples include ligand binding assays (i.e. potential medicine binding to a protein such as KIF1A), activity assays (i.e. testing potential medicines for improving KIF1A activity), and high-throughput screening assays (up to hundreds of thousands of potential drugs are tested for improvement in KIF1A function).
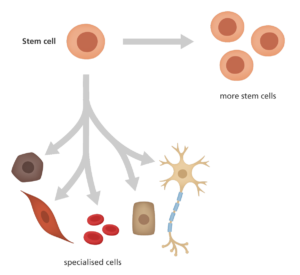
Stem cell: Most cells are specialized to carry out specific functions; however, stem cells are unspecialized cells that have the ability to develop into different types of cells in the body. They are unique for their ability to divide and renew themselves over a long period of time and become specialized. There are three main types of stem cells: embryonic stem cells, adult stem cells, and induced pluripotent stem cells. Embryonic stem cells can change into any cell in the body, and provide cells for a developing embryo. Adult stem cells provide new cells for growth and replace damaged ones. They are multipotent, which means they can change into a limited number of cells. Induced pluripotent stem cells are generated in a lab from normal adult cells. They are pluripotent, meaning they can develop into any type of cell. Stem cell therapy is the potential for stem cells to replace damaged cells, tissues and organs.
Induced pluripotent stem cells (iPSCs): Induced pluripotent stem cells are specialized cells that have been taken from the body and grown in petri dishes and then reprogrammed to become pluripotent (they can develop into any type of cell). They can be used as research to understand how different diseases develop, and are used as a tool in drug development. In addition, the cells can later be transplanted into the patient’s body because it is a perfect genetic match and avoids tissue matching or rejection issues.
Mouse model: Mouse models can represent human diseases and help scientists study how to treat and prevent them. Mice share 95% of human DNA. This means that mice and humans can be similarly affected by disease. As a result, by studying symptoms in mice, scientists can learn more about how to treat certain diseases.
Assay |Stem cell | Induced pluripotent stem cells (iPSCs) | Mouse model
