#ScienceSaturday posts share relevant and exciting scientific news with the KAND community. This project is a collaboration between KIF1A.ORG’s Research Engagement Team Leader Alejandro Doval, President Kathryn Atchley and Science Communication Director Dr. Dominique Lessard. Send news suggestions to our team at impact@kif1a.org.
Recent KIF1A-Related Research
Lysosomal Biology and Function: Modern View of Cellular Debris Bin
This week’s Recent KIF1A-Related Research discusses the topic of lysosomes, a type of waste removal system within our cells. Lysosomal vesicles are filled with digestive enzymes that help break down and recycle unused cellular components. In order for lysosomes to successfully recycle materials, they must be positioned and moved to the correct locations inside of a cell. Molecular motor proteins, including KIF1A, are responsible for the movements of lysosomes along microtubule roadways. Defective lysosomal trafficking and function has been observed in a variety of neurodegenerative disorders including Alzheimer’s, Huntington’s, and Parkinson’s disease. This review provides an in-depth overview of lysosomal roles and functions within our cells and what happens when this process goes awry.
Rare Disease News
Biochemists unveil molecular mechanism for motor protein regulation, insights into severe brain diseases
Dynein, like KIF1A, is an essential molecular motor protein involved in neuronal cargo transport. However, unlike KIF1A, dynein walks in the complete opposite direction along microtubule roadways! Dynein impairment has been linked to a neurodegenerative disease known as lissencephaly. Lissencephaly and KAND share many overlapping symptoms, such as seizures and limited motor function, highlighting the important role of molecular motors in our nervous system. Also similar to KAND, lissencephaly has been linked to mutations in a specific gene, in this case a gene known as LIS1. This article summarizes a recent discovery out of Steven Markus’ lab at Colorado State University that advances our understanding of the relationship between Lis1 and dynein function.
“I’m interested in the molecular basis for these diseases… there will be no therapeutic interventions without understanding how these molecules function… molecular motors are fun, because we can purify these motors and watch them walk on microtubules in real time…”
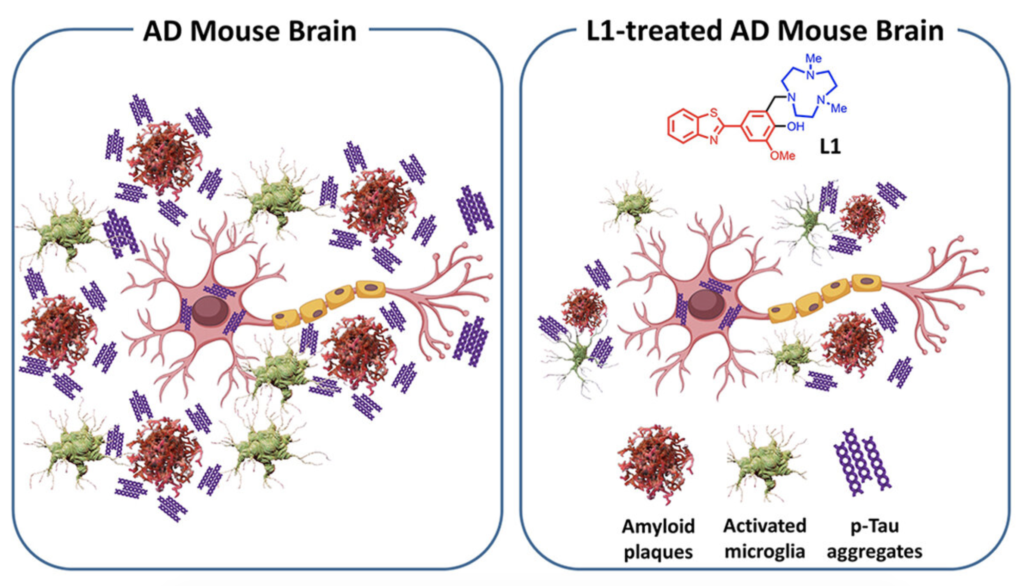
Molecule reduces multiple pathologies associated with Alzheimer’s disease
We have been talking a lot about a protein called “tau”- why is that? Tau is highly expressed protein in our brain that performs multiple roles needed to keep our nervous system functioning optimally. Because of this, we commonly find instances of tau dysfunction in neurodegenerative diseases, such as Alzheimer’s disease (covered in this article). One recent area of investigation by multiple research groups is focused on connecting dysfunctional tau, and other proteins, to an increased inflammatory state of the brain in neurodegenerative diseases. This article discusses one recent advancement by highlighting a new compound that has shown to reduce brain inflammation in an Alzheimer’s disease mouse model. Click the image below to read more!
New antagonists targeting misfolded RACK1 prevent dysfunctional protein aggregates from forming and restore normal function
Abnormal protein aggregation is a characteristic of many neurodegenerative diseases. When proteins are not expressed or recycled properly, they may begin to clump, or aggregate in our cells. These abnormal protein aggregates are typically very harmful to our nerve cells and thus have been a long-term drug target for treating neurodegenerative diseases. This article discusses a recent therapeutic development focused on relieving the aggregation of a protein known as RACK1.
“Evidence indicates that targeting RACK1 is a promising new strategy to address the complex mechanisms involved in the pathogenesis of neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS).”

