#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dominique Lessard and Dr. Dylan Verden of KIF1A.ORG summarize newly published KIF1A-related research and highlight progress in rare disease research and therapeutic development.
KIF1A-Related Research
End Binding Protein 1 promotes specific motor-cargo association in the cell body prior to axonal delivery of Dense Core Vesicles
A lot of logistics are involved in shipping a product: To arrive at your door it has to be packaged, transported safely along roads, and taken off of the delivery truck at the right time and place.
Of course, none of this works if the package doesn’t get loaded onto the truck in the first place. This is why shipping labels are so important; they tell us that this particular package needs to get on the truck heading south, not north.
It’s the same for the cargo carried by KIF1A: Once KIF1A picks up cargo in the neuron’s cell body, it needs instructions on where to go, and this depends on the type of cargo. In this week’s pre-print* researchers investigated a protein that tells KIF1A to leave the cell body and take cargo down the axon.
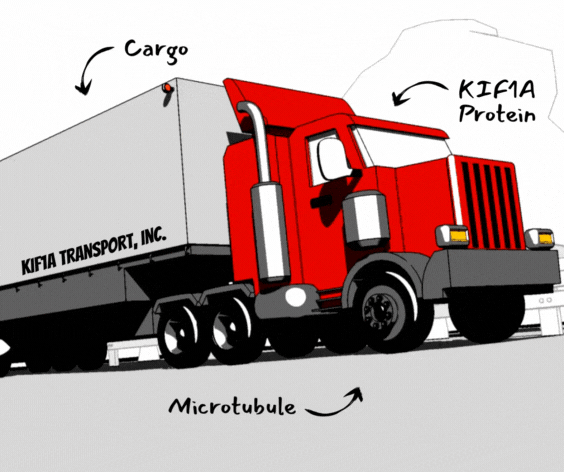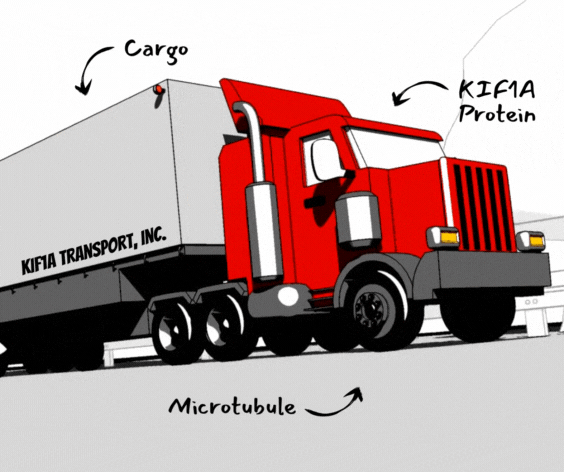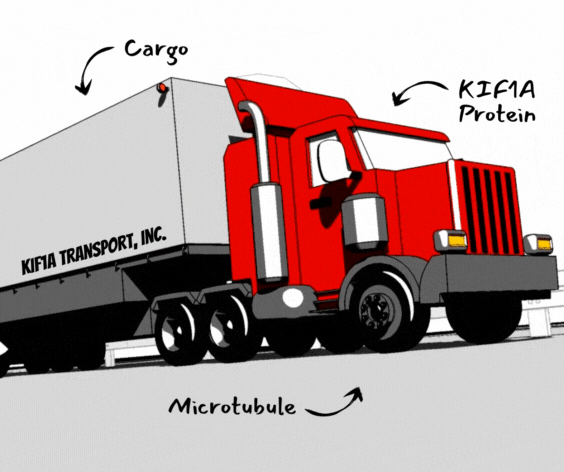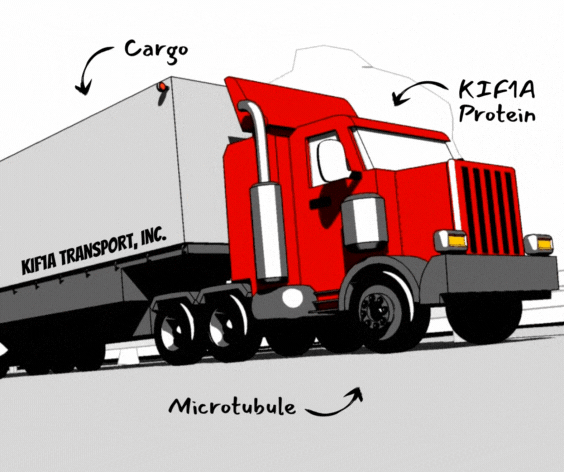
This protein is called End binding protein 1 (EB1), and it binds to microtubules and coordinates other proteins – it reads the shipping label on the cargo and helps it get on the right truck.
The researchers found that EB1 binds to KIF1A in neurons: When neurons lack EB1, a certain type of cargo called Dense Core Vesicles become stuck in the cell body because they aren’t shipped along the axon. This is important because the chemicals in Dense Core Vesicles are used to communicate between neurons.
By knocking out (i.e. removing) specific parts of the EB1 protein, the authors were able to determine that EB1 helps to attach Dense Core Vesicles to KIF1A so they can be shipped.
Because some KIF1A mutations prevent KIF1A from binding to cargo, we’re interested in proteins like EB1 that can regulate KIF1A’s interactions with its cargo. These proteins could be potential drug targets to help KIF1A load up its cargo more effectively.
*What’s a pre-print? Check out this #ScienceSaturday post to learn more
Rare Roundup
Hallmarks of neurodegenerative diseases
KAND is a neurodegenerative disease; so are Alzheimer’s, Parkinson’s, Multiple Sclerosis, and ALS. These diseases all have different symptoms, so what is a neurodegenerative disease?
Neurodegeneration is the loss of nervous system function over time. If this sounds like a broad definition, that’s because it is. While the death of neurons is the most obvious culprit, at a cellular level there are many factors that can cause this degeneration. This is important because strategies for “neuroprotection” and “neural repair” may differ between diseases, or may overlap. That’s why in a recent review, researchers identified hallmarks of neurodegenerative diseases – the cellular mechanisms that cause the nervous system to get worse.
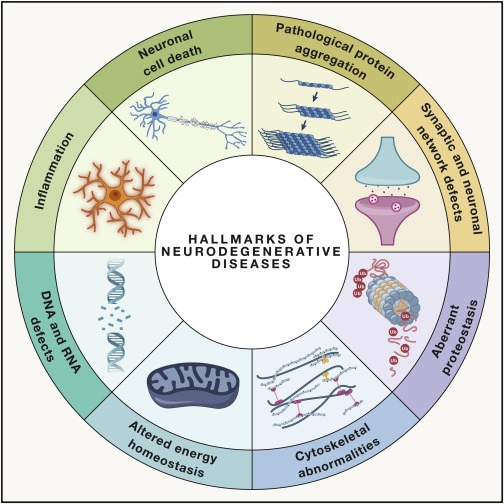
Each hallmark impacts neuronal health, and may require different strategies for treatment. For example, we know KIF1A mutations can cause neuronal network defects that contribute to spasticity and epilepsy, which is why our partners at NeuCyte and Murdoch Children’s Research Institute are hard at work investigating network activity in patient-derived neurons with KIF1A mutations. KIF1A mutations can also cause proteins to clump in axons which can damage neurons over time. But we know less about how other hallmarks like inflammation and energy deficits contribute to neurodegeneration in KAND, and these are areas that can benefit from more research.
By looking at these hallmarks of neurodegenerative diseases in general, we may be able to identify new targets and strategies to slow, prevent, or reverse damage to neurons in KAND, including those that are effective in other neurodegenerative diseases!

